Get the free PIPE Study - Randomized Controlled Trial of in ... - policyandorders cw bc
Show details
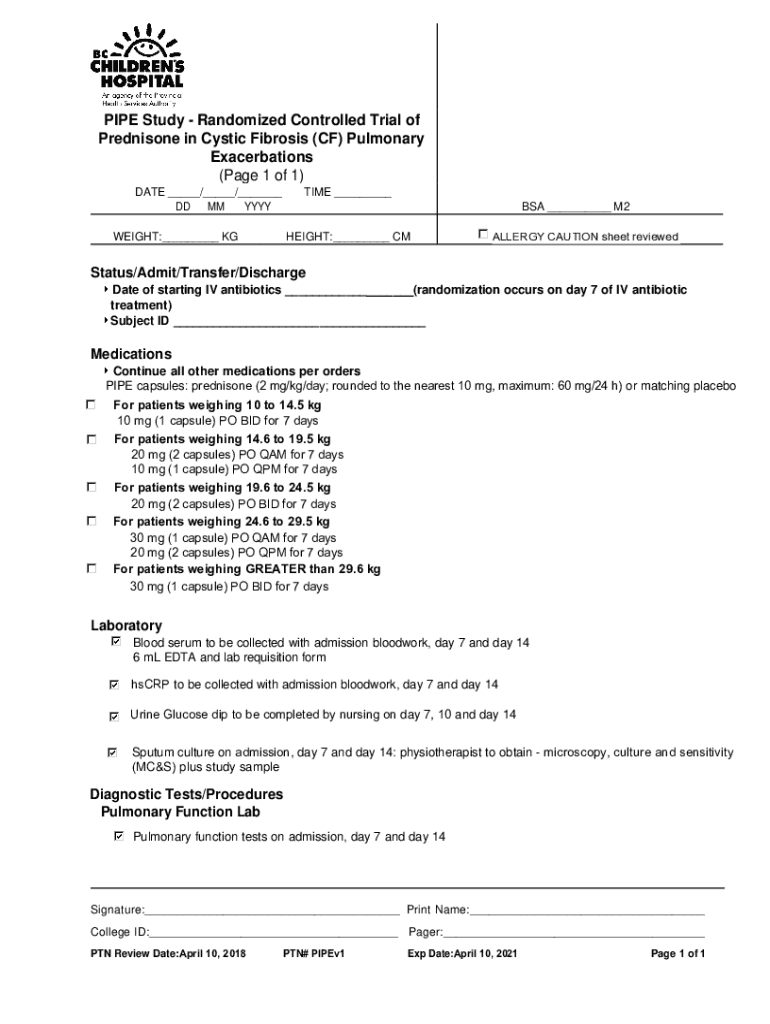
PIPE Study Randomized Controlled Trial of in Cystic Fibrosis (CF) Pulmonary Exacerbations (Page 1 of 1) DATE ___/___/___ DD MM DAYTIME ___ BSA ___ M2WEIGHT:___ HEIGHT:___ ALLERGY CAUTION sheet reviewedStatus/Admit/Transfer/Discharge
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign pipe study - randomized

Edit your pipe study - randomized form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your pipe study - randomized form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit pipe study - randomized online
To use the services of a skilled PDF editor, follow these steps below:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit pipe study - randomized. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out pipe study - randomized

How to fill out pipe study - randomized
01
Gather a list of participants who will be included in the study.
02
Randomly assign each participant to either the control group or the experimental group.
03
Ensure that the process for assigning participants to groups is truly random to avoid bias.
04
Conduct the study according to the established timeline and procedures.
05
Analyze the results of the study to determine if there is a significant difference between the two groups.
Who needs pipe study - randomized?
01
Researchers who are looking to test the effectiveness of a new intervention or treatment.
02
Policy makers who need evidence to support decision making.
03
Healthcare professionals who want to demonstrate the impact of a new procedure or therapy.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the pipe study - randomized electronically in Chrome?
Yes. By adding the solution to your Chrome browser, you can use pdfFiller to eSign documents and enjoy all of the features of the PDF editor in one place. Use the extension to create a legally-binding eSignature by drawing it, typing it, or uploading a picture of your handwritten signature. Whatever you choose, you will be able to eSign your pipe study - randomized in seconds.
How do I complete pipe study - randomized on an iOS device?
Install the pdfFiller app on your iOS device to fill out papers. If you have a subscription to the service, create an account or log in to an existing one. After completing the registration process, upload your pipe study - randomized. You may now use pdfFiller's advanced features, such as adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
How do I fill out pipe study - randomized on an Android device?
On an Android device, use the pdfFiller mobile app to finish your pipe study - randomized. The program allows you to execute all necessary document management operations, such as adding, editing, and removing text, signing, annotating, and more. You only need a smartphone and an internet connection.
What is pipe study - randomized?
Pipe study - randomized is a research study design in which participants are randomly assigned to different treatment groups to determine the efficacy of a particular intervention.
Who is required to file pipe study - randomized?
Researchers, scientists, or professionals conducting research studies in the field of medicine, psychology, or social sciences may be required to file pipe study - randomized.
How to fill out pipe study - randomized?
To fill out a pipe study - randomized, researchers need to carefully design the study protocol, recruit participants, randomly assign them to treatment groups, collect and analyze data, and report the findings.
What is the purpose of pipe study - randomized?
The purpose of pipe study - randomized is to determine the effectiveness of a particular intervention or treatment by comparing outcomes between different groups of participants.
What information must be reported on pipe study - randomized?
Researchers must report details of the study design, participant characteristics, intervention protocols, outcome measures, statistical analysis, and conclusions drawn from the study.
Fill out your pipe study - randomized online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pipe Study - Randomized is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















