Get the free Trial design - Field Trials of Health Interventions
Show details
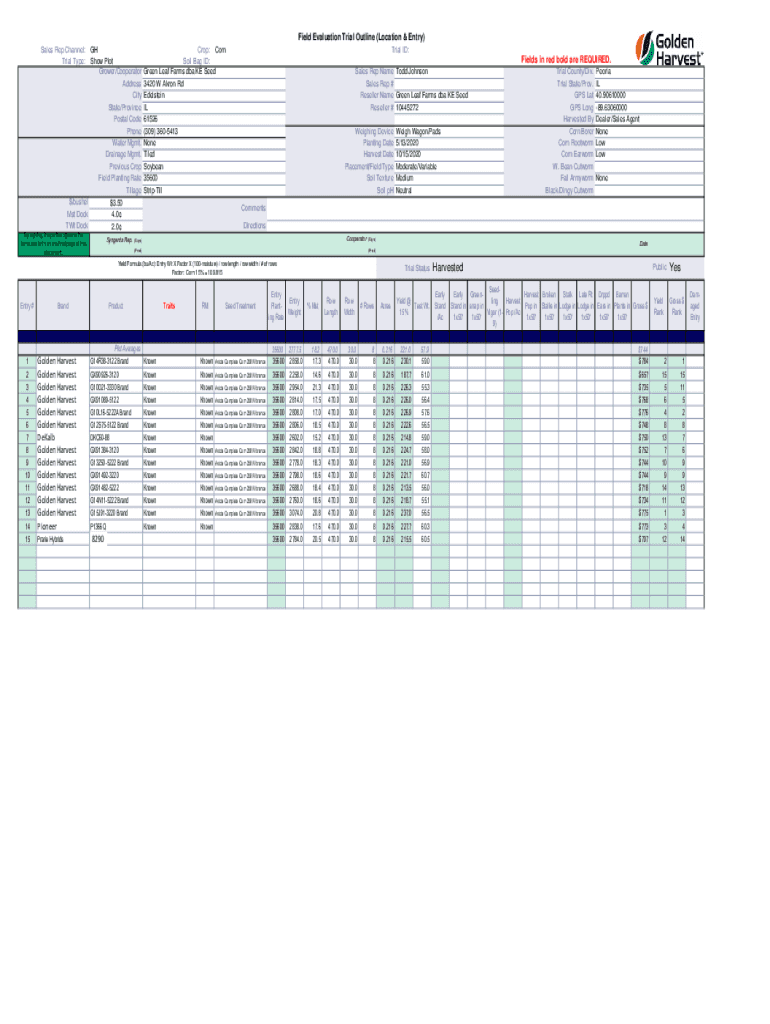
Field Evaluation Trial Outline (Location & Entry) Sales Rep Channel: Trial Type:GH Show Plot Grower/Cooperator Address City State/Province Postal Code Phone Water Mgmt. Drainage Mgmt. Previous Crop
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign trial design - field

Edit your trial design - field form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your trial design - field form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit trial design - field online
To use our professional PDF editor, follow these steps:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit trial design - field. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
The use of pdfFiller makes dealing with documents straightforward. Try it now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out trial design - field

How to fill out trial design - field
01
To fill out the trial design – field, follow the steps below:
02
Start by evaluating the purpose and objectives of the trial.
03
Determine the type of trial design that will best suit your research objectives. Common types include parallel, crossover, factorial, and sequential designs.
04
Gather the necessary information and data required to complete the trial design. This may include background research, statistical analysis, and sample size calculations.
05
Specify the trial interventions or treatments that will be tested. Provide detailed descriptions and instructions for each intervention.
06
Define the outcome measures that will be used to evaluate the effectiveness of the interventions. This may include clinical endpoints, patient-reported outcomes, or laboratory measurements.
07
Clearly outline the inclusion and exclusion criteria for participant eligibility. Specify any specific demographic or clinical characteristics that are required for inclusion.
08
Determine the randomization procedures that will be used to assign participants to treatment groups. Describe the methods and algorithms that will be employed.
09
Consider any ethical considerations or regulatory requirements that must be addressed in the trial design. This may include obtaining informed consent, ensuring patient privacy, and complying with relevant research guidelines.
10
Review and validate the trial design to ensure its feasibility and scientific validity. Seek input from colleagues, supervisors, or regulatory bodies if necessary.
11
Finally, document the trial design in a clear and concise manner. Use appropriate diagrams, tables, or flowcharts to illustrate the design if needed.
Who needs trial design - field?
01
The trial design – field is needed by researchers, scientists, and clinicians involved in planning and conducting clinical trials or research studies.
02
Clinical trial investigators rely on the trial design to determine the structure, procedures, and methods of their studies.
03
Pharmaceutical companies and drug developers utilize trial design to assess the safety and efficacy of new drugs or treatments.
04
Regulatory agencies such as the FDA or EMA require trial design documentation for reviewing and approving clinical trial protocols.
05
Ethics committees or institutional review boards (IRBs) also need the trial design to evaluate the ethical considerations and scientific merit of a proposed study.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in trial design - field without leaving Chrome?
trial design - field can be edited, filled out, and signed with the pdfFiller Google Chrome Extension. You can open the editor right from a Google search page with just one click. Fillable documents can be done on any web-connected device without leaving Chrome.
How do I edit trial design - field on an iOS device?
No, you can't. With the pdfFiller app for iOS, you can edit, share, and sign trial design - field right away. At the Apple Store, you can buy and install it in a matter of seconds. The app is free, but you will need to set up an account if you want to buy a subscription or start a free trial.
How do I complete trial design - field on an Android device?
Use the pdfFiller mobile app and complete your trial design - field and other documents on your Android device. The app provides you with all essential document management features, such as editing content, eSigning, annotating, sharing files, etc. You will have access to your documents at any time, as long as there is an internet connection.
What is trial design - field?
Trial design - field refers to the structured approach used in conducting trials, particularly in testing and evaluating products, processes, or interventions in various fields.
Who is required to file trial design - field?
Typically, organizations or entities conducting trials, including research institutions, companies, and individual researchers, are required to file trial design - field.
How to fill out trial design - field?
To fill out trial design - field, one must provide detailed information about the trial's objectives, methodology, participant selection, data collection methods, and anticipated outcomes.
What is the purpose of trial design - field?
The purpose of trial design - field is to ensure that trials are conducted systematically and ethically, yielding credible and valid results that can be communicated to stakeholders.
What information must be reported on trial design - field?
Information that must be reported includes trial objectives, methodology, participant criteria, locations, duration, outcome measures, and data analysis plans.
Fill out your trial design - field online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Trial Design - Field is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















