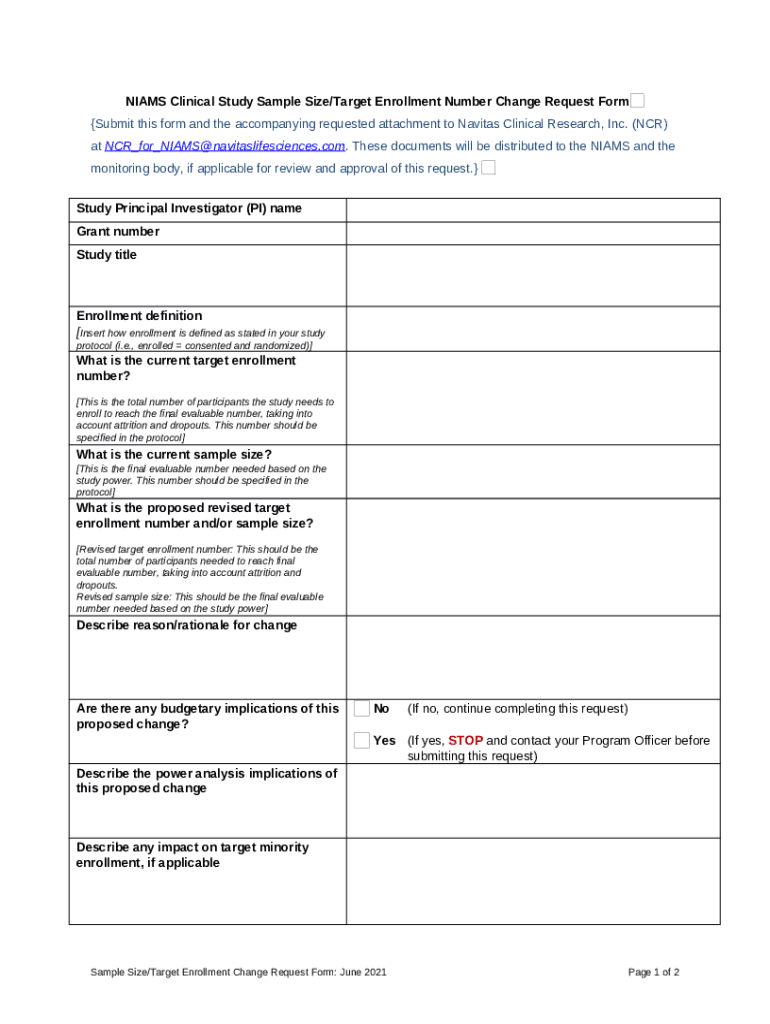
What is NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli Form?
The NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli is a document that should be submitted to the specific address to provide some info. It has to be completed and signed, which can be done manually in hard copy, or via a particular solution like PDFfiller. It allows to complete any PDF or Word document right in the web, customize it depending on your purposes and put a legally-binding e-signature. Right away after completion, you can send the NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli to the relevant person, or multiple recipients via email or fax. The editable template is printable as well because of PDFfiller feature and options offered for printing out adjustment. Both in electronic and in hard copy, your form will have a organized and professional outlook. Also you can save it as the template for further use, without creating a new blank form from scratch. All that needed is to customize the ready form.
NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli template instructions
Prior to start filling out the NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli fillable template, you have to make certain all the required info is well prepared. This part is highly important, as long as errors and simple typos can lead to unpleasant consequences. It is really unpleasant and time-consuming to re-submit forcedly the entire word form, letting alone the penalties came from blown deadlines. To handle the figures requires more concentration. At first glance, there is nothing tricky in this task. Nevertheless, it doesn't take much to make a typo. Professionals recommend to save all required information and get it separately in a different document. Once you've got a writable sample, you can easily export it from the file. Anyway, all efforts should be made to provide true and correct information. Check the information in your NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli form twice when completing all necessary fields. You can use the editing tool in order to correct all mistakes if there remains any.
NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli: frequently asked questions
1. Would it be legit to file documents digitally?
As per ESIGN Act 2000, forms written out and authorized using an electronic signature are considered as legally binding, equally to their physical analogs. This means that you can fully fill out and submit NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli form to the establishment required to use electronic solution that fits all requirements according to certain terms, like PDFfiller.
2. Is my personal information secured when I fill out forms online?
Yes, it is totally safe when you use reliable product for your work flow for such purposes. As an example, PDFfiller provides the benefits like:
- All personal data is stored in the cloud provided with multi-level encryption, and it's prohibited from disclosure. It's user only who has access to data.
- Every word file signed has its own unique ID, so it can’t be forged.
- User can set extra security such as verification of signers by photo or password. There's also an option to secure entire folder with encryption. Place your NIAMS Clinical Study Sample Size/Target Enrollment Number Change Request . Submit this and the accompanying requested attachment to Navitas Clinical Research. These documents will be distributed to the NIAMS and the monitoring body, if appli word template and set a password.
3. How can I upload required data to the fillable form from another file?
Yes, but you need a specific feature to do that. In PDFfiller, we call it Fill in Bulk. By using this feature, you are able to export data from the Excel worksheet and insert it into your file.