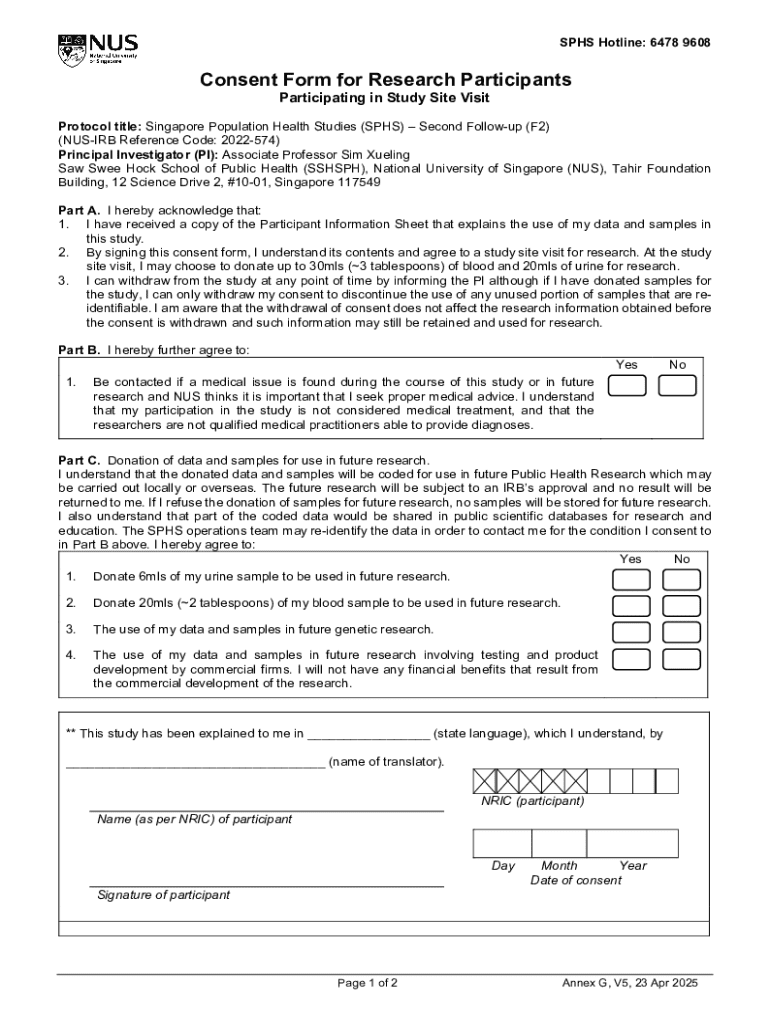
Get the free Consent Form for Research Participants
Get, Create, Make and Sign consent form for research



Editing consent form for research online
Uncompromising security for your PDF editing and eSignature needs
How to fill out consent form for research

How to fill out consent form for research
Who needs consent form for research?
Understanding Consent Forms for Research
Understanding research consent forms
A research consent form is a vital document in the ethics of research involving human participants. It serves as a declaration of the participant's agreement to partake in a study after being adequately informed about its details. Ensuring informed consent is crucial to uphold ethical standards and respect participants’ autonomy.
The concept of consent in research ethics is paramount; it underscores the rights of individuals to make informed choices about their participation. Typically, the research consent form includes detailed information about the study's purpose, procedures, potential risks, and benefits to both participants and broader society.
Regulatory requirements impact how consent is obtained and documented. For instance, 45 CFR 46 mandates that institutions adhere to ethical guidelines for the protection of human subjects, ensuring that consent forms fulfill necessary legal and ethical criteria.
Key components of a research consent form
A comprehensive research consent form consists of several key components that collectively ensure participants are fully informed before consenting to participate in a study.
Types of consent forms
Various types of consent forms exist catering to different research contexts. Identifying the appropriate form type is essential for compliance and ethical integrity.
Considerations for special populations
Certain populations require additional considerations when it comes to consent due to their vulnerability or specific legal requirements. Researchers must navigate these situations delicately to ensure ethical compliance.
Creating a consent form with pdfFiller
Using pdfFiller streamlines the process of creating and managing research consent forms. With a variety of customizable templates, you can ensure that your consent form is compliant and conveys all necessary information.
Here are the steps you can follow to create a compelling consent form:
Filling out a research consent form
Researcher attention to detail is vital when completing a consent form. Each section must be filled out accurately to uphold ethical standards and protect participants’ rights.
To correctly complete each section of the form, consider the following tips:
Reviewing and finalizing consent forms
Reviewing consent forms before use is essential. This process helps to ensure the accuracy and completeness of information provided to participants, thus maintaining trust and complying with regulations.
A checklist for completeness may include:
Examples and templates
Having access to varied consent form templates can greatly facilitate the process of developing your own documents. pdfFiller offers downloadable templates that cater to various types of research.
Reviewing case studies of real-world applications of consent forms in research can also provide critical insights and inspiration for structure and language.
Additional tools and guides
To further support researchers in developing robust consent forms, pdfFiller provides an array of checklists and FAQs. These resources are created to simplify the consent form development process and address common inquiries about research consent.
Navigating the consent process
Streamlining the consent process is critical for both researchers and participants. With pdfFiller's features, researchers can enhance their workflows and ensure clarity in communication.
Quick reference tips include:






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit consent form for research on an iOS device?
How do I complete consent form for research on an iOS device?
How do I fill out consent form for research on an Android device?
What is consent form for research?
Who is required to file consent form for research?
How to fill out consent form for research?
What is the purpose of consent form for research?
What information must be reported on consent form for research?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















