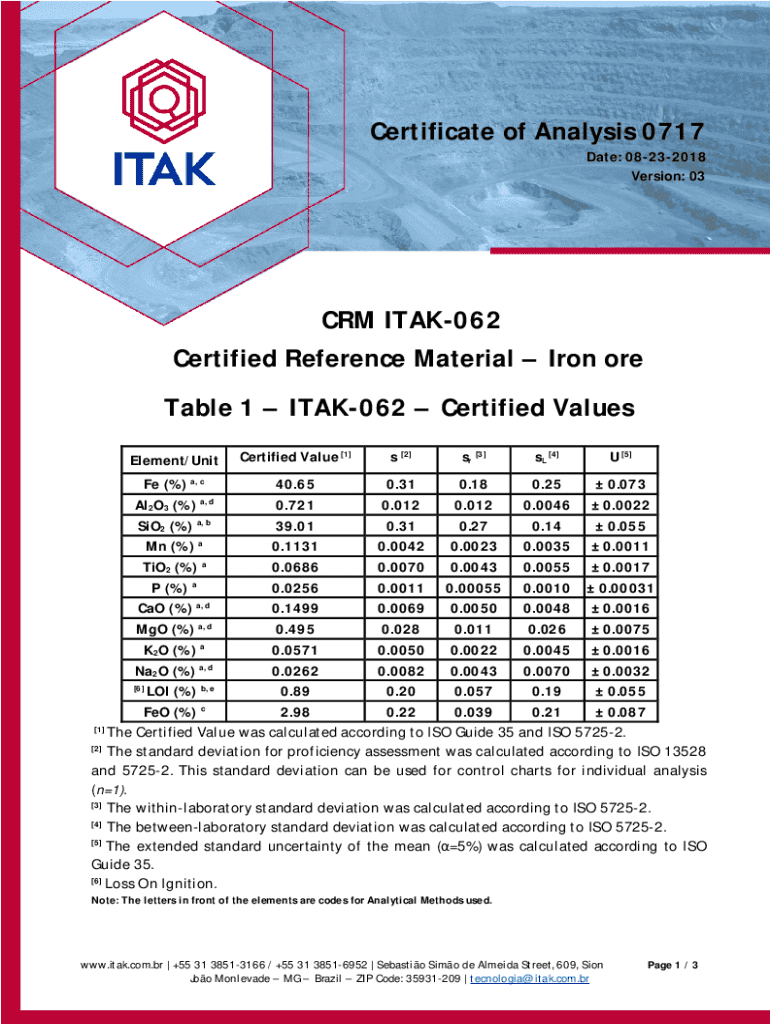
Get the free Certificate of Analysis 0717
Get, Create, Make and Sign certificate of analysis 0717



Editing certificate of analysis 0717 online
Uncompromising security for your PDF editing and eSignature needs
How to fill out certificate of analysis 0717

How to fill out certificate of analysis 0717
Who needs certificate of analysis 0717?
A comprehensive guide to the certificate of analysis 0717 form
Understanding the certificate of analysis 0717 form
The certificate of analysis (CoA) 0717 form serves as an essential document that verifies the quality and purity of materials, particularly in industries where compliance and safety are paramount. It provides a comprehensive overview of testing outcomes performed on samples, ensuring that these materials meet required standards and specifications. Essentially, the CoA acts as proof that a product has been tested and that it conforms to safety, quality, and regulatory standards.
The importance of a Certificate of Analysis cannot be overstated. It plays a critical role in quality control processes, helping organizations confirm that incoming materials meet predetermined quality benchmarks before being used in production. Additionally, many regulatory bodies require a CoA as part of compliance documentation, thus highlighting its importance across diverse sectors, including pharmaceuticals, food and beverage, cosmetics, and chemical manufacturing.
Key elements of the certificate of analysis 0717 form
The Certificate of Analysis 0717 form contains several key elements crucial for its validity and utility. First and foremost is the sample identification, which includes unique identifiers for the sample being tested, such as batch numbers or specimen ID. This section ensures traceability and accountability in quality assurance processes.
Next, the test results section presents a comprehensive breakdown of various analytical tests conducted on the sample, including quantitative and qualitative assessments. It often includes metrics such as purity levels, potency, microbiological data, and other vital parameters that establish compliance with industry standards. Furthermore, signatures and certifications from authorized personnel validate the report, confirming that the testing has been carried out according to regulatory norms.
The process of completing the certificate of analysis 0717 form
Completing the Certificate of Analysis 0717 form requires careful attention to detail to ensure that all information is accurate and compliant. The process typically begins with gathering necessary information regarding the sample and the tests that need to be conducted. This could include details about the testing methodology, required parameters, and relevant regulatory documents.
Once the information is collected, the next step is filling out the form. This involves providing accurate and thorough details in each section of the form, such as specifying the sample’s batch number, the testing date, and the corresponding results. Clear guidelines on entering test results facilitate efficient completion, ensuring no vital information is omitted. Validation follows, emphasizing the importance of double-checking all entries for errors or discrepancies. To finalize the form, it must be signed by authorized personnel, which can now be accomplished through electronic signing options to maintain digital compliance.
Editing and managing your certificate of analysis 0717 form
Post-completion, efficient management of the Certificate of Analysis 0717 form is crucial for maintaining records and ensuring accessibility. Tools like pdfFiller enable users to edit PDF forms effectively. Features such as highlighting, note-taking, and making corrections streamline the editing process, allowing users to update information as needed while retaining the original context of the document.
In addition to editing capabilities, storing completed forms in the cloud adds another dimension to document management. This ensures that authorized team members can easily access documents from anywhere at any time. It effectively minimizes the risk of losing critical paperwork and also facilitates quick retrieval for audits or regulatory inspections.
Collaborating on the certificate of analysis 0717 form
Collaboration plays a vital role in managing the Certificate of Analysis 0717 form effectively, especially for teams working in quality control and compliance. With pdfFiller, users can invite team members to contribute to the form, allowing multiple stakeholders to input necessary data relevant to their expertise. This collective input ensures a comprehensive document free from oversights or incorrect information.
Moreover, pdfFiller’s real-time collaboration features enhance productivity. Team members can work simultaneously on the document, making it easier to track changes made by different users. Version management is another critical aspect, as it allows teams to maintain an organized record of changes and updates—providing transparency and facilitating the review process.
Common mistakes to avoid when completing the certificate of analysis 0717 form
When filling out the Certificate of Analysis 0717 form, attention to detail is critical to avoid common pitfalls. One frequent mistake is errors in data entry, such as typos or misrecorded test results. Even minor mistakes can compromise the integrity of the document, leading to compliance issues or product recalls.
Additionally, misinterpretation of test results can occur, particularly if individuals lack the necessary expertise to analyze data adequately. It is crucial to ensure that those completing the form have a proper understanding of the testing processes. Finally, failing to meet regulatory standards, such as skipping required tests or certifications, can lead to serious repercussions. Regular training and adherence to regulatory guidelines can help prevent these discrepancies.
Tips for effective use of the certificate of analysis 0717 form
To ensure the Certificate of Analysis 0717 form is utilized effectively, consider implementing best practices that enhance accuracy and compliance. One major tip is to create a standardized procedure for filling out the CoA, including checklists that ensure every necessary element is completed. This approach minimizes the risk of overlooking significant details.
Furthermore, leveraging automation tools can provide efficiency and reinforce compliance. Automated reminders for required testing, data entry stipulations, and compliance monitoring can guide teams effectively throughout the process. Additionally, maintaining an accessible archive of past CoA records can serve as a useful reference for future analyses, fostering continual improvement in quality assurance practices.
Case studies: successful management of certificate of analysis forms
Several industries have successfully implemented efficient management systems for the Certificate of Analysis forms, demonstrating the practical application of tools like pdfFiller. For instance, a pharmaceutical company was able to streamline their testing documentation process by using pdfFiller to collaborate on CoAs among different departments. This significantly reduced the time required to get products approved for distribution.
Another example is found within the food and beverage sector, where a major manufacturer utilized pdfFiller to enhance their quality control operations by allowing real-time edits and updates to their CoAs. The ability to track changes ensured ongoing compliance with health and safety regulations, ultimately resulting in a more transparent quality guarantee process. These case studies illustrate how leveraging digital tools can empower organizations to manage compliance documentation more effectively.
FAQs about the certificate of analysis 0717 form
Frequent questions regarding the Certificate of Analysis 0717 form often revolve around its requirements and best practices. For instance, users may wonder about the necessary components that must be included on the form. Essential sections include sample identification, test results, and the signatures of authorized personnel. Home users often ask how to ensure the CoA remains compliant with regulatory standards, necessitating a clear understanding of local and international testing requirements.
Another common query pertains to the accessibility of completed forms. Many users wish to know how to store and retrieve these documents effortlessly within their organizations. Utilizing cloud-based platforms, like pdfFiller, can provide seamless access and storage solutions. Regularly reviewing and updating your procedures regarding the CoA process will ensure all stakeholders remain informed and compliant.
Related documents and templates
When managing quality and compliance documentation, it is beneficial to understand the various types of Certificates of Analysis, along with customizable templates available through pdfFiller. Other variants of CoA, such as those tailored to different industries like food safety or cosmetic regulations, provide specific frameworks that meet their respective compliance standards.
Additionally, creating custom templates with pdfFiller enhances efficiency and ensures clarity in documentation. Tailoring templates to specific products or testing methods allows teams to streamline their documentation processes effectively while adhering to all necessary regulatory requirements.
Staying updated on regulatory changes affecting certificate of analysis
Staying informed about regulatory changes affecting the Certificate of Analysis is essential for compliance and operational integrity. Regularly reviewing updates from relevant authorities, participation in industry conferences, and engaging with professional organizations can provide valuable insights into regulatory modifications. Organizations should proactively adjust their practices according to new guidelines to ensure they are always aligned with the latest standards.
Additionally, resources such as regulatory announcements, newsletters, and dedicated compliance training sessions can help organizations effectively navigate the complexities of evolving industry standards. Allocating time and resources for ongoing education fosters a culture of compliance and keeps teams prepared for audits and assessments.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the certificate of analysis 0717 in Chrome?
Can I create an eSignature for the certificate of analysis 0717 in Gmail?
How can I fill out certificate of analysis 0717 on an iOS device?
What is certificate of analysis 0717?
Who is required to file certificate of analysis 0717?
How to fill out certificate of analysis 0717?
What is the purpose of certificate of analysis 0717?
What information must be reported on certificate of analysis 0717?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















