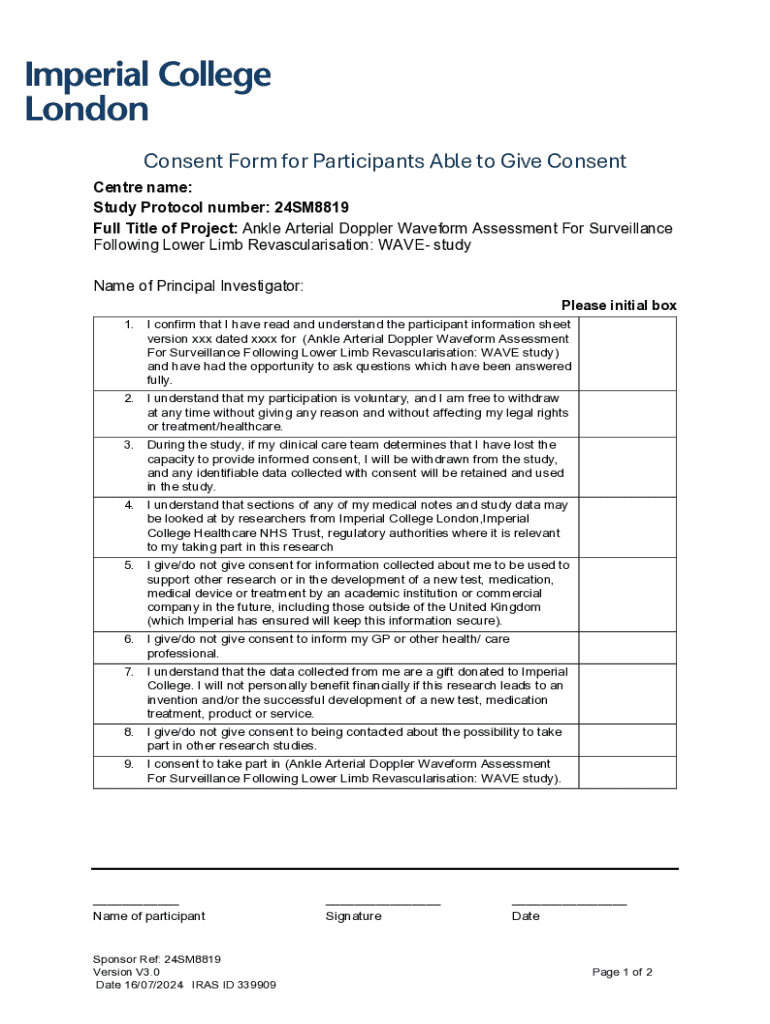
Get the free Consent Form for Participants Able to Give Consent
Get, Create, Make and Sign consent form for participants



Editing consent form for participants online
Uncompromising security for your PDF editing and eSignature needs
How to fill out consent form for participants

How to fill out consent form for participants
Who needs consent form for participants?
Comprehensive Guide to Consent Form for Participants
Understanding consent forms
A consent form for participants is a crucial document that outlines the terms and conditions under which individuals agree to partake in a study, event, or activity. The primary purpose of these forms is to ensure that participants are fully informed about what their involvement entails. This includes details on how their data will be used, the nature of the participation, and any associated risks.
In various contexts, from clinical trials to educational research and event planning, obtaining explicit informed consent helps participants make knowledgeable decisions. This aligns with ethical standards and legal requirements, contributing to the integrity of any undertaking involving human subjects.
Types of consent forms for participants
Different scenarios require specific consent forms tailored to their unique demands. General consent forms provide a broad template that can be adapted across various fields, while research participation consent forms delve deeper into specifics necessary for studies. Each serves a distinct purpose while maintaining similar foundational elements.
In medical contexts, consent forms must clearly articulate the nature of the treatment or procedure as well as any potential risks. When minors are involved, obtaining parental permission becomes critical, adhering to legal standards that ensure the safety and welfare of the young participants. Waiver request forms apply in situations where a participant must agree to relinquish certain rights or claims, adding another layer of complexity.
Key components of a consent form
A well-constructed consent form must contain essential elements that facilitate understanding and protect participants' rights. Key components include a clear statement of purpose, which outlines what the study or activity involves, as well as any risks and potential benefits associated with participation.
Additionally, confidentiality measures are vital in ensuring that personal information is safeguarded. It's equally important to highlight participants’ rights, including the ability to withdraw consent at any time. Finally, the form should include signature and date fields for verification purposes, formally establishing the participant's agreement.
Steps to create a consent form for participants
Creating an effective consent form involves a structured approach. The first step is to identify the purpose of the form and recognize your audience. This helps frame the language appropriately and ensures relevance.
Next, drafting the content requires clear and concise language. Avoiding ambiguity is crucial; every term should be understandable. Using existing templates can save time but should be customized to ensure they meet specific requirements of your study or activity.
After completing the draft, it’s essential to review and revise it. This step may involve feedback from peers to enhance clarity and comprehensiveness. Once revisions are made, finalizing the document is next, paying attention to formatting for professional presentation and ensuring compliance with relevant regulations.
Tools for managing consent forms
pdfFiller offers robust tools for creating and managing consent forms seamlessly. This platform empowers users to design documents tailored to specific needs. Features include an intuitive interface that allows for easy editing and formatting of consent forms, making it accessible for both individuals and teams.
For participant interaction, pdfFiller provides interactive options that facilitate engagement with the forms. eSigning capabilities enable participants to sign documents electronically, ensuring a smooth process for document completion and reducing delays often associated with physical signatures. Collaboration features enhance teamwork, allowing multiple stakeholders to make necessary form revisions while keeping the document secure.
Common mistakes to avoid
When drafting consent forms, several pitfalls can undermine their effectiveness. Incomplete information is a primary error; omitting critical fields can lead to misunderstandings or even legal challenges. Making assumptions about what participants understand can lead to ambiguity in the form’s language, which may result in confusion regarding the consent being provided.
Additionally, ignoring legal guidelines when creating consent forms can lead to non-compliance with regulations. It’s crucial to stay informed about local and federal laws governing consent, ensuring that the document adheres to best practices. Taking the time to avoid these common mistakes will enhance the clarity, legal standing, and overall effectiveness of the consent form.
Real-world applications and case studies
Numerous case studies illustrate the importance of well-crafted consent forms. For instance, in clinical research, comprehensive consent forms helped to elucidate study risks, leading to increased participant trust and engagement. Gathering feedback from these participants revealed that clarity in consent language significantly impacted their understanding and comfort with the processes.
Similarly, educational programs requiring parental consent successfully minimized liability issues when clear forms were employed explaining student involvement. Success stories highlight how thorough attention to consent form details not only protects participants but also fosters an environment of transparency and respect.
Frequently asked questions (FAQs)
When it comes to consent forms, participants often have questions about the validity and handling processes. One common inquiry is what happens if a participant revokes consent after signing a form. The general practice allows participants to revoke consent at any time, which must be documented appropriately.
Handling consent forms from international participants can also raise questions; it's critical to understand the legal frameworks applicable in different jurisdictions, as these can vary significantly. Furthermore, many users wonder if electronic consent forms are legally binding. In most regions, eSignatures are recognized as valid, provided that certain conditions are met, ensuring that participants' consent is upheld.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete consent form for participants online?
How can I edit consent form for participants on a smartphone?
How do I fill out consent form for participants on an Android device?
What is consent form for participants?
Who is required to file consent form for participants?
How to fill out consent form for participants?
What is the purpose of consent form for participants?
What information must be reported on consent form for participants?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















