Get the free Chapter 2: Dalton's Atomic Theory
Get, Create, Make and Sign chapter 2 daltons atomic



Editing chapter 2 daltons atomic online
Uncompromising security for your PDF editing and eSignature needs
How to fill out chapter 2 daltons atomic

How to fill out chapter 2 daltons atomic
Who needs chapter 2 daltons atomic?
Chapter 2: Dalton's Atomic Form
Overview of Dalton’s atomic theory
John Dalton introduced his Atomic Theory in the early 1800s, laying the groundwork for modern chemistry. His theory is significant as it provided a systematic explanation of the nature of matter based on atoms, marking a shift from philosophical speculation to a scientific approach. This transition was crucial during a time dominated by debates over the nature of elements and compounds.
Preceding Dalton were figures like Democritus, who proposed the idea of indivisible atoms, and Antoine Lavoisier, whose work on conservation of mass established essential principles that Dalton would build upon. In this historical milieu, Dalton sought to provide empirical evidence for the existence of atoms, which had been largely theoretical.
Core component of Dalton’s atomic form
Dalton’s atomic form conceptualizes the atom as the smallest unit of matter, indivisible and uniform in size. Each element consists of unique atoms that differ from those of other elements in mass and properties. This foundational idea positions atoms at the heart of chemical reactions, where they combine in fixed ratios to form compounds.
At the core of Dalton's theory are his postulates: 1) Matter is made of atoms, 2) Atoms are indivisible, 3) Atoms of the same element are identical, 4) Compounds form from combinations of different atoms, and 5) A chemical reaction involves rearranging atoms. These postulates were revolutionary; they provided a clear framework that challenged older, less systematic models of matter.
Key modifications to Dalton’s original theory
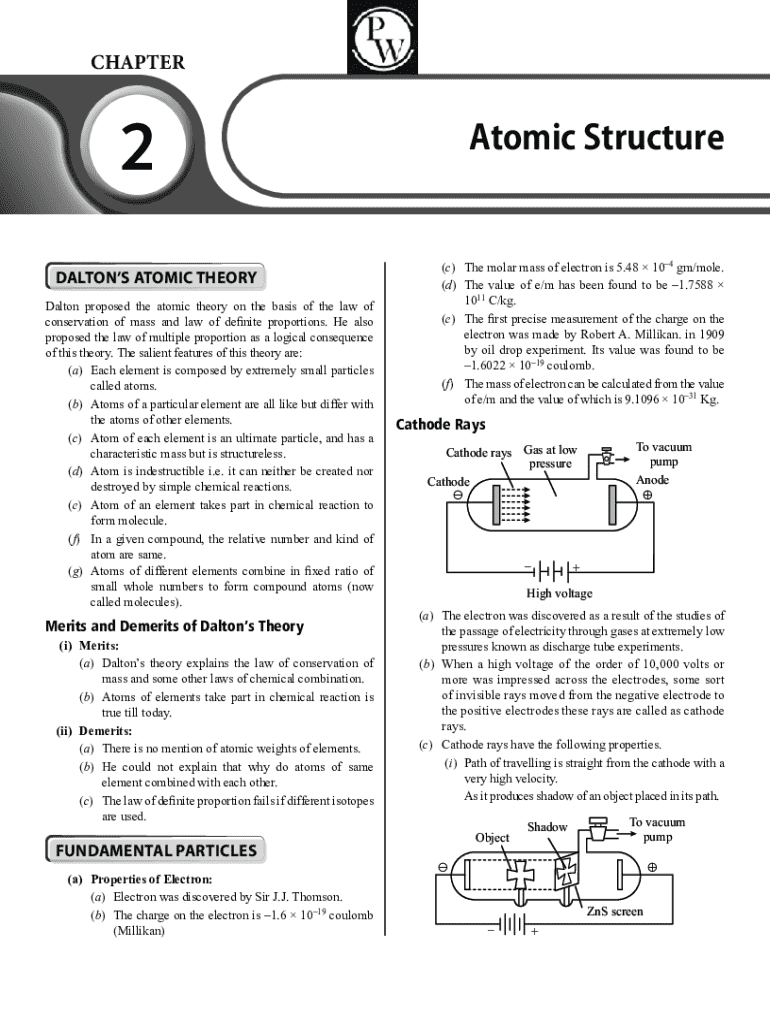
While Dalton's theory was groundbreaking, it was later discovered that atoms are indeed divisible. The discovery of subatomic particles—electrons, protons, and neutrons—revolutionized our understanding of atomic structure. Electrons, with their negative charge, were discovered through J.J. Thomson's cathode ray experiments, leading to the realization that atoms are not the fundamental building blocks once thought.
1) Electrons carry a negative charge and are found outside the nucleus. 2) Protons, which are positively charged, reside in the nucleus, while neutrons carry no charge. 3) These particles possess varying masses and charges, which contributed significantly to the development of more sophisticated atomic models, such as Rutherford's nuclear model.
Experiments such as the Gold Foil Experiment further demonstrated that atoms have structured components, leading to a more nuanced understanding of chemical behavior. These modifications highlight the dynamic nature of scientific inquiry and the importance of experimentation.
Formation of compounds: Dalton’s perspective
Dalton posited that atoms combine to form compounds in fixed ratios, a principle reflected in his interpretation of the Law of Multiple Proportions. This law states that when two elements combine to form more than one compound, the mass ratio of the elements in the compounds is always a simple whole number. For example, carbon can combine with oxygen to form CO and CO2, illustrating these concepts in nature.
Visualizing atomic combinations can aid in understanding these interactions. **Examples include**: - Water (H2O): Two hydrogen atoms bond with one oxygen atom. - Ammonia (NH3): One nitrogen atom bonds with three hydrogen atoms. Interactive tools available today help students simulate these combinations, enhancing their grasp of molecular structures.
Impacts of Dalton's atomic form on modern chemistry
Dalton's atomic form paved the way for subsequent atomic models and theories, shaping the landscape of chemistry as we know it. His work laid a foundation for significant experiments that validated and sometimes challenged his concepts, leading to advancements in understanding chemical behavior and interactions. Notably, experiments in the late 19th and early 20th centuries further confirmed and refined theories about atomic structure.
For instance, Avogadro's Hypothesis, which states that equal volumes of gases contain equal numbers of molecules under the same conditions, built on Dalton's ideas. As chemists explored atomic theory, they implemented Dalton’s principles to elucidate gaseous behavior, catalyzing discoveries in various fields such as thermodynamics and quantum chemistry.
Visualizing atomic structure
To comprehend Dalton's atomic model, diagrams contrasting it with modern understandings can be particularly effective. In Dalton’s model, atoms are represented as solid spheres without internal structure—simple and elegant for its time. In contrast, contemporary depictions exhibit complex structures with nucleus and cloud models, illustrating the dynamic nature of electron positioning.
Interactive elements, such as simulations showcasing atomic interactions, serve to enrich the learning experience. These tools allow users to visualize electron orbits, atomic bonding, and even chemical reactions, which can significantly enhance understanding and engagement with the material.
Common questions and clarifications
Several questions frequently arise when exploring Dalton's atomic form. Notably, the concept of whether atoms can be created or destroyed prompts discussion. According to the law of conservation of mass, atoms are neither created nor destroyed in chemical reactions, but they can change forms. This principle underlines the fundamental nature of atomic interactions.
Another inquiry revolves around isotopes—atoms of the same element with different atomic masses. Isotopes conform to Dalton's framework as they possess the same number of protons but vary in neutron count, showcasing atomic diversity while maintaining elemental identity.
Experiments that shaped atomic theory
Several pivotal experiments have sharpened our atomic theory insights. Key among these is Rutherford's Gold Foil Experiment, which revealed that an atom's mass and charge are concentrated in a small nucleus, fundamentally altering previous assumptions about atomic structure. This experiment contradicted Dalton's indivisible atom model, showing instead a complex internal organization.
Equally influential was Thomson's Cathode Ray Experiment, which identified electrons and established the idea of subatomic particles, challenging the completeness of Dalton’s original theories. Together, these experiments contributed to an enhanced understanding of how matter is structured, driving scientific inquiry forward.
Interactive tools to explore atomic theory
Engaging with atomic theory can be made easier through various interactive tools. **pdfFiller, for instance**, offers features to create and manage documents associated with atomic theory, making it simple for educational institutions and study groups to collaborate effectively.
These tools allow users to fill out, edit, sign, and manage research papers or projects related to atomic theory in a collaborative environment. A step-by-step guide is available on how to utilize these document tools to research and present findings on Dalton's Atomic Theory.
Practical application and relevance today
Dalton's atomic form continues influencing various branches of modern chemistry, including organic, inorganic, and physical chemistry. For instance, pharmaceuticals develop compounds based on atomic interactions that stem from Dalton's principles of atomic combination and ratios.
Case studies demonstrating Dalton’s principles are evident in drug synthesis, material science, and environmental chemistry. Understanding how atoms interact provides crucial insights for real-world applications like developing new materials and creating medications, showcasing the theory's ongoing relevance.
Glossary of key terms related to Dalton's atomic theory
To facilitate understanding, here’s a glossary of key terms related to Dalton’s Atomic Theory: - **Atom**: The smallest unit of an element that retains its properties. - **Element**: A substance composed of identical atoms. - **Compound**: A substance formed from two or more different types of atoms. - **Postulate**: A fundamental assumption or principle. Interactive activities that test knowledge of these terms can also help reinforce concepts.
Learning objectives and outcomes
Studying Dalton's atomic theory equips learners with essential insights into the building blocks of matter. Core objectives include understanding the model of the atom, recognizing the significance of subatomic particles, and grasping how atoms combine to form compounds. The goals extend to mastering the implications of Dalton's ideas in modern science.
Overall, this knowledge fosters a comprehensive appreciation of chemistry's evolution and prepares individuals to contribute meaningfully to discussions around molecular interactions and atomic principles.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete chapter 2 daltons atomic online?
How do I make edits in chapter 2 daltons atomic without leaving Chrome?
Can I create an eSignature for the chapter 2 daltons atomic in Gmail?
What is chapter 2 daltons atomic?
Who is required to file chapter 2 daltons atomic?
How to fill out chapter 2 daltons atomic?
What is the purpose of chapter 2 daltons atomic?
What information must be reported on chapter 2 daltons atomic?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















