Get the free Cdisc Operating Procedure 002
Get, Create, Make and Sign cdisc operating procedure 002



Editing cdisc operating procedure 002 online
Uncompromising security for your PDF editing and eSignature needs
How to fill out cdisc operating procedure 002

How to fill out cdisc operating procedure 002
Who needs cdisc operating procedure 002?
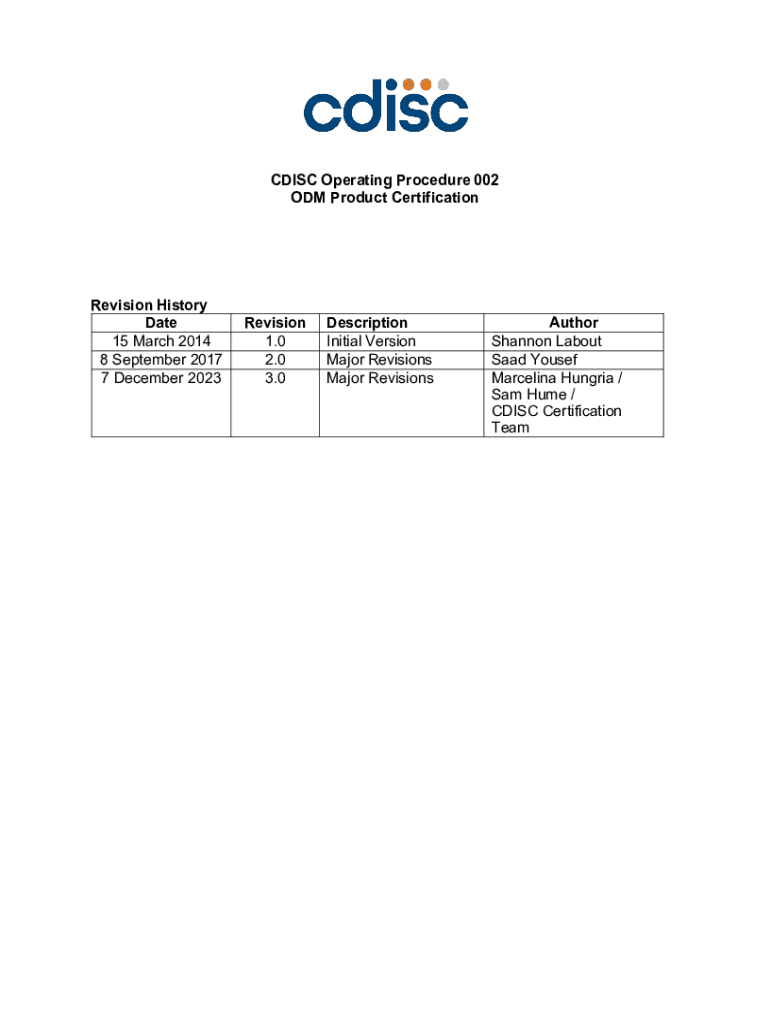
CDISC Operating Procedure 002 Form: A Comprehensive Guide
Understanding the CDISC Operating Procedure 002 Form
The CDISC Operating Procedure 002 Form is a crucial document used in clinical research to ensure that studies adhere to standardized protocols and procedures established by the Clinical Data Interchange Standards Consortium (CDISC). This form serves as a systematic approach for documenting key aspects of clinical trials, facilitating data sharing, and promoting consistency across different studies.
Compliance with the CDISC Operating Procedure 002 Form is vital for regulatory approvals and data integrity. As clinical research continues to evolve, adherence to established data standards helps researchers, sponsors, and regulatory bodies manage and interpret clinical data effectively.
CDISC standards provide a framework for organizing and reporting clinical research data. By following these guidelines, researchers can ensure their findings are easily interpretable and shareable within the scientific community.
Key components of the CDISC Operating Procedure 002 Form
The CDISC Operating Procedure 002 Form consists of several essential components that provide structure and clarity. Understanding its breakdown is instrumental for accurate completion and compliance.
Most of the form's fields fall into either required or optional categories. While the mandatory fields must always be completed to satisfy regulatory demands, the optional fields may provide additional useful context.
Common terminologies used in the form
The CDISC Operating Procedure 002 Form contains specific terminology that is vital for proper understanding. A glossary of key terms ensures all stakeholders are aligned in their comprehension of the document.
Step-by-step instructions for filling out the CDISC Operating Procedure 002 Form
Completing the CDISC Operating Procedure 002 Form requires careful attention to detail. Here’s a structured approach to ensure all necessary data is provided accurately.
Preparing to complete the form
Before diving into filling out the form, it's crucial to gather all necessary information. This includes participant details, study specifics, and the data standards you plan to implement. Familiarization with applicable regulatory requirements is also essential to ensure compliance.
Maintaining a checklist of required documents can streamline the process and minimize errors during form completion.
Detailed filling instructions
For an accurate and compliant submission, it’s advisable to have someone review your completed form for clarity and correctness before finalization.
Editing and modifying the CDISC Operating Procedure 002 Form
Editing the CDISC Operating Procedure 002 Form is a common task during the clinical research process. Whether it’s a minor correction or a major update, understanding the editing capabilities is key.
Version history tools also allow for tracking changes, which is vital for maintaining data integrity during editing.
eSigning the CDISC Operating Procedure 002 Form
eSigning the CDISC Operating Procedure 002 Form offers significant advantages in ensuring speed and efficiency in the clinical research process. Digital signatures simplify workflows and maintain the integrity of the document.
Collaborating on the CDISC Operating Procedure 002 Form
Collaboration is essential in clinical research, particularly when multiple team members are involved in completing the CDISC Operating Procedure 002 Form. PdfFiller provides robust tools that facilitate teamwork.
Managing and storing the CDISC Operating Procedure 002 Form
Proper management and storage of the CDISC Operating Procedure 002 Form is vital to maintaining data security and compliance. PdfFiller’s cloud-based solutions offer a strategic advantage.
Archiving completed forms is equally important, ensuring historical data is preserved for future reference and regulatory audits.
Troubleshooting common issues with the CDISC Operating Procedure 002 Form
Encountering issues with the CDISC Operating Procedure 002 Form can be frustrating, but understanding common pitfalls can lead to swift resolutions.
Additional considerations for CDISC procedure forms
Staying updated on regulations and guidelines from relevant regulatory bodies enhances compliance and effectiveness in clinical trials. Institutions like the FDA and EMEA often release updates that influence CDISC standards and procedures.
You may also like
Expand your knowledge and efficiency with additional resources linked to the CDISC Operating Procedure. Explore related forms and templates specifically designed to enhance your clinical research capabilities.
Engaging with our community
Join our community to stay at the forefront of clinical research developments. Sign up for our newsletter to receive the latest updates, participate in webinars, and provide feedback on how we can improve.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify cdisc operating procedure 002 without leaving Google Drive?
How do I execute cdisc operating procedure 002 online?
How do I fill out cdisc operating procedure 002 using my mobile device?
What is cdisc operating procedure 002?
Who is required to file cdisc operating procedure 002?
How to fill out cdisc operating procedure 002?
What is the purpose of cdisc operating procedure 002?
What information must be reported on cdisc operating procedure 002?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















