Get the free Cdisc Protocol Controlled Terminology, 2019-06-28
Get, Create, Make and Sign cdisc protocol controlled terminology



How to edit cdisc protocol controlled terminology online
Uncompromising security for your PDF editing and eSignature needs
How to fill out cdisc protocol controlled terminology

How to fill out cdisc protocol controlled terminology
Who needs cdisc protocol controlled terminology?
CDISC Protocol Controlled Terminology Form - How-to Guide
Understanding CDISC Protocol Controlled Terminology
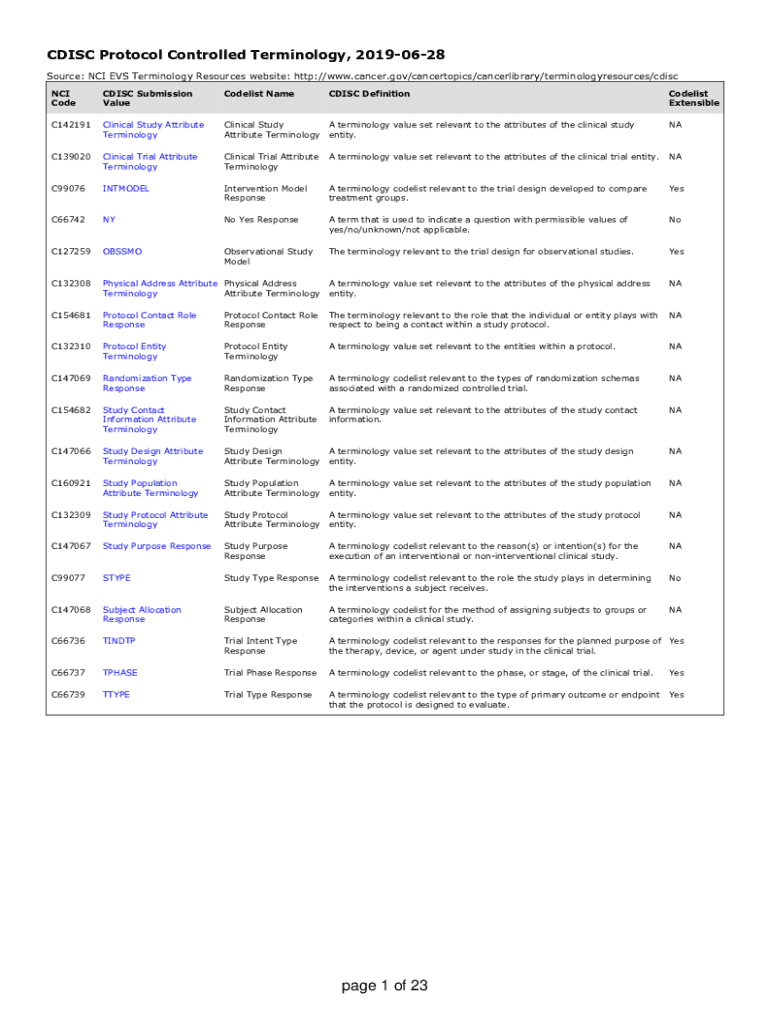
The Clinical Data Interchange Standards Consortium (CDISC) is a global initiative aimed at establishing data standards in clinical research. The goal is to ensure consistency and quality of data across various studies and organizations. By utilizing CDISC standards, clinical researchers can enhance the efficiency and effectiveness of data sharing. This standardization is crucial in a field where data integrity can fundamentally impact patient safety and research outcomes.
Controlled terminology plays a pivotal role within this framework. Defined as a set of terms used consistently to describe clinical data, it ensures all stakeholders interpret the data uniformly. This consistency mitigates the risks of miscommunication and data misinterpretation, critical factors in the regulatory landscape of clinical trials.
The importance of using the CDISC Protocol Controlled Terminology Form
Using the CDISC Protocol Controlled Terminology Form is paramount for data standardization in clinical trials. This standardized vocabulary enables researchers to present data in a consistent manner, simplifying data aggregation and analysis. When all participants in a trial adhere to the same terminology, the complexities of data integration across multiple sources are significantly reduced. This consistency is essential, especially in multi-center studies where disparate systems might be employed.
Furthermore, using this controlled terminology aligns with regulatory requirements. Regulatory agencies, such as the FDA and EMA, expect clinical trial data to meet specific standards, including the use of proper terminology. By utilizing the CDISC terminology, sponsors can streamline the submission process and enhance the clarity of their data packages. This approach not only facilitates quicker review times but also reduces the likelihood of regulatory questions.
Accessing and navigating the CDISC Protocol Controlled Terminology Form
Accessing the CDISC Protocol Controlled Terminology Form has been streamlined through platforms like pdfFiller. To start using the form, you need to visit the pdfFiller website and search for the CDISC Protocol Controlled Terminology Form. The user-friendly interface allows for easy navigation, ensuring that you can locate the form without hassle.
Once on the pdfFiller platform, creating a user account will provide access to various features. After logging in, you'll find a user account menu that allows you to manage your documents, view previously saved forms, and access additional tools for document editing and collaboration.
Filling out the CDISC Protocol Controlled Terminology Form
Filling out the CDISC Protocol Controlled Terminology Form is straightforward when you follow a structured approach. Start by carefully reviewing the form requirements and each section’s objective. The form typically includes sections for identifying the study, specifying patient characteristics, and detailing data collection methods.
Accurate data entry is crucial; hence, using drop-down menus as guided by the controlled terminology enhances consistency. Pay attention to the defined terms, ensuring they match your data collection criteria. Additionally, always double-check your entries to avoid common mistakes like mislabeling terms or omitting critical information.
Editing and managing your CDISC Protocol Controlled Terminology Form
Editing the CDISC Protocol Controlled Terminology Form can be efficiently handled within the pdfFiller interface. If you need to make changes after initial completion, simply access your saved form, and utilize the intuitive editing tools available. You can modify text, update selections, and even add annotations as necessary.
Collaboration is often essential in these scenarios. PdfFiller facilitates this by allowing you to share the form with teammates or stakeholders easily. They can review, comment, and suggest changes in real-time, which significantly enhances the workflow and ensures that multiple perspectives are considered.
eSigning the CDISC Protocol Controlled Terminology Form
Electronic signatures are becoming increasingly vital in clinical documentation, including when submitting the CDISC Protocol Controlled Terminology Form. ESigning allows for a more streamlined approach, eliminating the need for physical signatures and ensuring rapid processing of documents. This electronic approach complies with regulations set forth by governing bodies, making it a viable option for modern clinical research.
Using pdfFiller, eSigning the form is a straightforward process. After completing your form, there is an option to add an electronic signature directly within the platform. This guarantees that your documents are legally binding and can be securely transmitted to relevant parties without delay.
FAQ section for CDISC Protocol Controlled Terminology Form users
Users often have questions about the CDISC Protocol Controlled Terminology Form and its related processes. A common inquiry is about the specific clinical terms utilized in the form and how to align them with current studies. Misunderstandings about the scope and applications of controlled terminology are prevalent, and addressing these can help ensure compliance and effectiveness in data management.
In case users encounter issues within the platform, providing a comprehensive troubleshooting guide is paramount. It's also beneficial to have a clear process for submitting feedback or new term requests to foster continuous improvement.
Additional features of pdfFiller for enhanced document management
Utilizing pdfFiller extends beyond simply managing the CDISC Protocol Controlled Terminology Form. The platform’s cloud-based tools enable users to efficiently manage documents across multiple devices while ensuring data accessibility. Users can create, edit, and share documents seamlessly, making it an excellent solution for teams working in collaborative environments.
Security is a top concern when handling sensitive clinical data. PdfFiller incorporates various security features—such as encryption and secure storage—to safeguard your information and ensure compliance with data protection regulations. Knowing that comprehensive security measures are in place gives users peace of mind when handling confidential information.
Understanding future developments in CDISC controlled terminology
Staying updated on changes in CDISC controlled terminology is essential for all researchers in clinical trials. Recent updates have introduced new terms and revisions to existing definitions, reflecting the ongoing evolution in the field of clinical research. Keeping abreast of these changes ensures that all stakeholders use the most current and precise terminology in their studies.
Researchers can actively participate in the development and updates of controlled terminology as CDISC encourages community feedback. Engaging with the CDISC community provides an avenue to contribute to the improvement of standards and practices within clinical trials, ensuring that the terminology used remains relevant and useful.
Staying connected with the CDISC community
Engagement with the CDISC community is crucial for professionals in clinical research. By following the organization's forums, social media accounts, and newsletters, users can gain insights into ongoing initiatives, educational resources, and updates on controlled terminology. This network not only fosters knowledge sharing but also creates opportunities for professional development.
For those seeking more information on controlled terminology, the CDISC website offers comprehensive resources, including documentation and best practices, further enriching the understanding and application of the CDISC Protocol Controlled Terminology Form in clinical trials.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in cdisc protocol controlled terminology?
Can I edit cdisc protocol controlled terminology on an iOS device?
Can I edit cdisc protocol controlled terminology on an Android device?
What is cdisc protocol controlled terminology?
Who is required to file cdisc protocol controlled terminology?
How to fill out cdisc protocol controlled terminology?
What is the purpose of cdisc protocol controlled terminology?
What information must be reported on cdisc protocol controlled terminology?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















