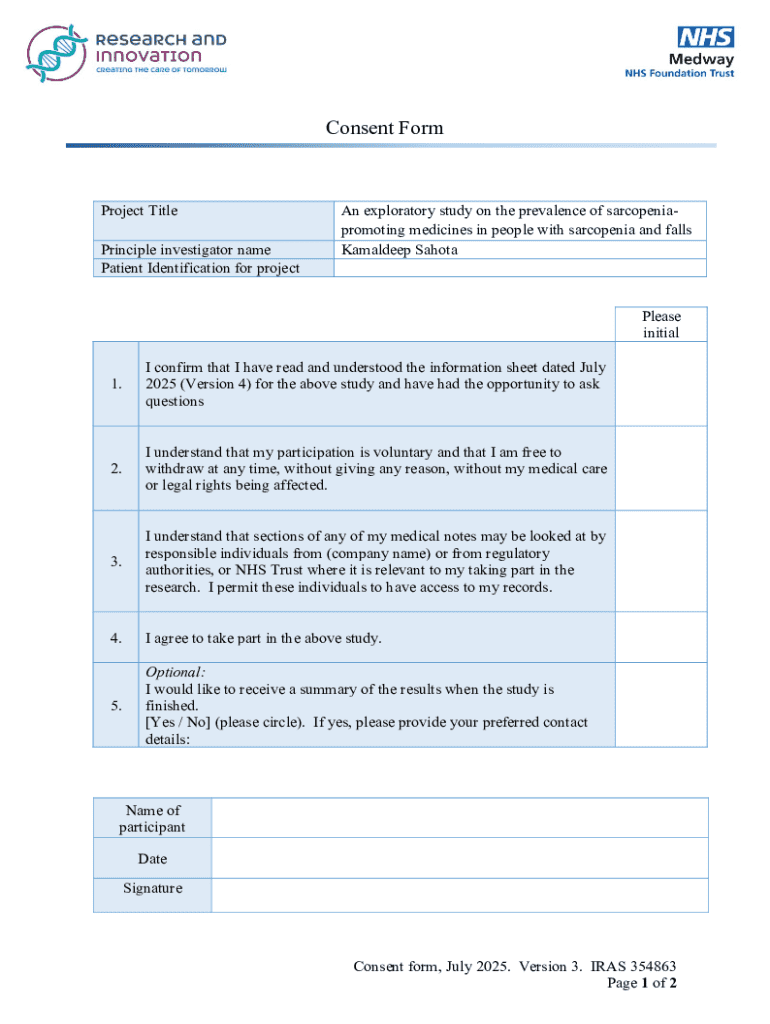
Get the free Consent Form
Get, Create, Make and Sign consent form



Editing consent form online
Uncompromising security for your PDF editing and eSignature needs
How to fill out consent form

How to fill out consent form
Who needs consent form?
A comprehensive guide to consent forms: Understanding, crafting, and managing
Understanding consent forms
A consent form is a vital document that outlines key information, ensuring that individuals are fully informed before agreeing to participate in any activity. These forms play a significant role in various contexts such as medical procedures, research studies, or legal agreements. Understanding the importance of consent forms means recognizing their purpose—not just as a legal formality, but as a cornerstone for ethical practices. This includes safeguarding individuals' rights and ensuring transparency in interactions.
Moreover, consent forms carry legal implications that can significantly affect an organization’s operations. They ensure compliance with relevant laws and regulations and help protect against possible legal disputes. For instance, when engaging in experimental research, it is imperative to obtain informed consent from participants to both meet ethical standards and fulfill legal requirements.
Types of consent forms
Consent forms can be categorized into several types, tailored for various situations. General consent forms are perhaps the most common, used across numerous fields to obtain permission before proceeding with an activity. These forms typically encompass standard elements such as the purpose of the consent, risks, benefits, and the right to withdraw, ensuring clarity and understanding for participants.
Parental permission and child assent forms are crucial when minors are involved. These forms address specific requirements and legal considerations, emphasizing the need for clear communication with parents or guardians. Furthermore, special consent forms can apply in contexts such as research studies approved prior to August 2021, and unique circumstances involving expanded access to medical devices. Each type of consent form serves essential functions tailored to the needs of the participants and the nature of the consent.
Key elements of an effective consent form
To create an effective consent form, clarity and readability are pivotal. The use of plain language ensures that participants can easily comprehend the document without getting lost in legal jargon or complex terms. Structuring information logically allows for a straightforward flow of understanding, guiding participants through key points of the consent effectively.
Moreover, an effective consent form includes essential components that clearly outline the purpose, risks, benefits, and procedures to handle data privacy. A critical aspect is obtaining freely given, specific, informed, and unambiguous consent from participants. The importance of affirmative action—from the direct input of the participant in signing—cannot be overstated, as it ensures compliance and legitimacy in the consent obtained.
Crafting your consent form
Writing a consent form requires strategic planning and execution. Start by identifying the specific purpose the consent is meant to serve—this foundational step shapes the details included in the form. Following this, include all relevant details, such as what participants can expect, any associated risks, and who to contact for questions or concerns.
Legal compliance is non-negotiable, so reviewing the document against applicable laws and ethical guidelines is essential. Once the draft is complete, seeking feedback from stakeholders enhances the content's clarity and ensures all perspectives, especially those of participants, have been considered.
Common mistakes can be made during this process, including vague language which can result in misunderstandings, as well as a lack of transparency about anticipated risks. Simplicity in wording is key to a document’s effectiveness. Additionally, reviewing sample consent forms ensures the inclusion of all necessary components and can guide the structure of your tailored version.
Digital consent tools and solutions
In our increasingly digital world, managing consent forms has never been easier than with tools like pdfFiller. This platform empowers users to seamlessly edit PDFs, sign documents, and collaborate with stakeholders from any location. The editing features facilitate quick adjustments, ensuring forms remain current and relevant.
eSigning integration enables rapid approval processes, eliminating the need for physical interactions that can slow down operations. Utilizing interactive tools through pdfFiller allows users to customize consent forms efficiently. Organizations can choose templates that suit their needs while harnessing options for multi-user collaboration, leading to cohesive, well-managed consent document workflows.
Managing consent forms
Once consent forms are created, effective management practices must be established for storage and sharing. Ensuring data security is paramount, especially when handling sensitive information. Following compliance guidelines, such as the GDPR, ensures that both participants' rights and organizational accountability are maintained.
Another critical aspect of consent management is understanding how to safely withdraw consent. Clear procedures and documentation are needed to respect participants’ rights while keeping organizations accountable. Establishing straightforward, concise guidelines for withdrawing consent helps participants feel safe and informed about the process.
Additional considerations in consent management
Consistently reviewing your consent forms is necessary to ensure they reflect current practices and legal requirements. Factors influencing the need for updates include changes in laws, the evolving landscape of research ethics, or the introduction of new technologies. Keeping consent forms relevant and up-to-date reinforces trust with participants.
The role of informed consent is especially critical in research. Ethical research practices revolve around respect for participants’ autonomy and the necessity of transparency in what participation involves. Frequent FAQs about consent forms can arise from both participants and institutions, emphasizing the need for clear communication and accessible answers to common queries.
Navigating consent form regulations
Understanding the regulatory frameworks governing consent forms is vital. Regulations vary widely based on jurisdictions, including national laws and international guidelines. Organizations must stay aware of legal changes to keep consent forms compliant with national and international standards.
Maintaining awareness of legislation changes can involve subscribing to industry updates, attending relevant workshops or seminars, and checking with governance bodies such as the Office of Human Research Protection. These resources equip organizations with the knowledge necessary to ensure their consent forms remain in line with evolving legal obligations.
Conclusion on effective consent management
Effective management of consent forms establishes a foundation of trust and transparency between organizations and participants. Investing time and resources into creating, reviewing, and updating consent forms cultivates a responsible and ethical operational framework. Ongoing education for team members regarding consent processes increases understanding and facilitates smoother interactions.
Ultimately, leveraging tools like pdfFiller can simplify the process of managing consent documents. Empowering teams to create, edit, sign, and collaborate on forms from a centralized platform enhances efficiency and ensures legal compliance, reaffirming commitment to ethical practices in any environment.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify consent form without leaving Google Drive?
How do I edit consent form online?
How do I make edits in consent form without leaving Chrome?
What is consent form?
Who is required to file consent form?
How to fill out consent form?
What is the purpose of consent form?
What information must be reported on consent form?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















