Get the free Potentially Hazardous Biological Agents Risk Assessment Form (6a)
Get, Create, Make and Sign potentially hazardous biological agents



Editing potentially hazardous biological agents online
Uncompromising security for your PDF editing and eSignature needs
How to fill out potentially hazardous biological agents

How to fill out potentially hazardous biological agents
Who needs potentially hazardous biological agents?
Understanding the Potentially Hazardous Biological Agents Form
Understanding potentially hazardous biological agents
Potentially hazardous biological agents encompass a range of microorganisms and toxins that can pose significant risks to human health and safety. These agents can be categorized according to their biological nature and potential for harm. Classification typically includes infectious agents, such as bacteria, viruses, and parasites, as well as non-infectious agents like toxins.
Infectious agents are organisms capable of causing diseases. Common examples include bacteria like Salmonella, viruses such as Influenza, fungi like Candida, and parasites, including Plasmodium. Toxins, produced either by biological cells or chemical processes, can have debilitating effects even in small quantities, making them a serious concern in research and public health.
Importance of the potentially hazardous biological agents form
The potentially hazardous biological agents form is a critical component of biosafety and regulatory compliance in research settings. This form acts as an official declaration of intent to handle or experiment with dangerous biological agents, ensuring that individuals and institutions adhere to biosecurity regulations.
Several regulatory bodies, including the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), provide guidelines for the safe handling and use of these agents. Complying with these guidelines not only protects researchers but also safeguards public health by minimizing the risk of exposure or accidental release.
Overview of the potentially hazardous biological agents form structure
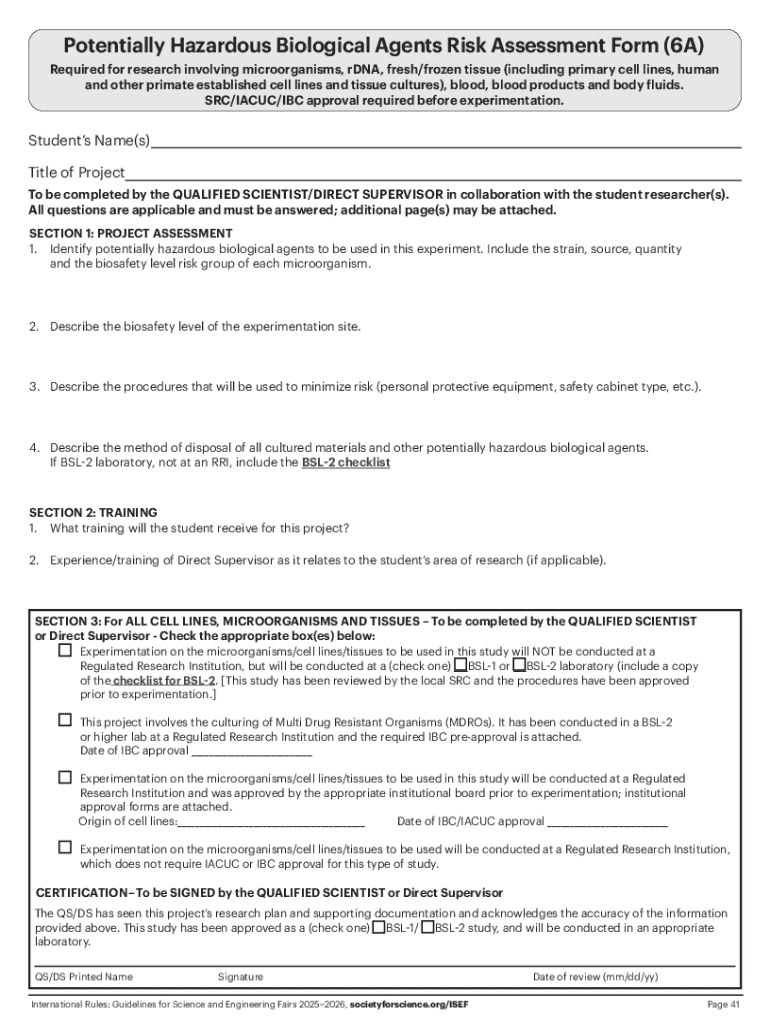
The structure of the potentially hazardous biological agents form is designed to streamline the submission process while capturing essential information about the agent and its associated risks. This form generally consists of several key sections that collectively help in risk assessment and management.
Common sections include an identification segment that records the researcher's personal and institutional details, a description of the biological agent which outlines its specific nature, and a risk assessment level that communicates how dangerous the agent is based on established criteria. Each section requires thorough completion to ensure compliance.
Steps for filling out the form
Before completing the potentially hazardous biological agents form, it is crucial to gather all necessary information and materials. This preparation involves consulting knowledgeable authorities or compliance officers who can clarify specific requirements and expectations for form submission.
Once you're ready to fill out the form, follow this step-by-step guide for accurate completion:
Editing and submitting the form
Accuracy is of utmost importance when documenting potentially hazardous agents, as mistakes can lead to legal issues or safety breaches. Best practices for reviewing submissions include utilizing tools like pdfFiller for editing and signing parts of the documentation electronically. This platform simplifies the process, enhancing efficiency and collaborative editing.
After careful review and completion of the form, submit it either electronically or in hard copy. The guidelines provided by your institution will typically specify the preferred submission methods, and upon submission, expect a review period where your form will be evaluated for compliance.
Tracking and managing your submission
Once you have submitted the potentially hazardous biological agents form, utilizing pdfFiller's cloud-based solutions can significantly enhance your workflow. This platform provides advantages such as document management and tracking capabilities, ensuring that you can monitor the status of your submission closely.
With pdfFiller, you can easily access and modify submitted forms, which is particularly useful for ongoing research projects. The platform's version control and history tracking features allow you to maintain accurate records of all changes made.
Compliance and ongoing obligations
Post-submission, it is vital to engage in follow-up actions to maintain compliance with biosafety regulations. This includes required safety training for personnel involved in handling hazardous agents, as well as adherence to regular compliance checks to ensure that all laboratory practices align with regulatory standards.
Collaboration with Institutional Review Boards (IRBs) should be ongoing, maintaining open communication about safety protocols and updates on research findings. This relationship is essential in addressing any safety concerns proactively.
Interactive tools and resources
pdfFiller enhances the process of handling potentially hazardous biological agents forms with interactive features designed for ease of use. This includes tools for form completion, digital signature capabilities, and intelligent document management, allowing teams to collaborate effectively.
Additionally, users can access valuable resources from regulatory bodies, including links to CDC and NIH guidelines. These resources often provide access to training materials and compliance checklists, assisting institutions in meeting safety requirements.
Real-world case studies and examples
Understanding the practical implications of the potentially hazardous biological agents form is illustrated through case studies of institutions successfully navigating its requirements. These examples highlight institutions that have developed effective protocols for managing hazardous agents while ensuring compliance with regulatory requirements.
These institutions have learned from past submission processes, identifying common pitfalls such as incomplete information or inadequate risk assessments, thereby refining their approach to submitting forms in the future.
Future of research and handling of biological agents
The landscape of research involving potentially hazardous biological agents is continuously evolving. Emerging trends indicate a shift towards incorporating innovative safety protocols that leverage technology, improving compliance and safety outcomes in laboratories.
Technological advancements play a crucial role in enhancing research safety management. From automated compliance checks to smart tracking systems, these tools not only streamline processes but also reduce the burden on researchers, allowing them to focus on their projects while maintaining high safety standards.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify potentially hazardous biological agents without leaving Google Drive?
How do I fill out potentially hazardous biological agents using my mobile device?
How do I complete potentially hazardous biological agents on an iOS device?
What is potentially hazardous biological agents?
Who is required to file potentially hazardous biological agents?
How to fill out potentially hazardous biological agents?
What is the purpose of potentially hazardous biological agents?
What information must be reported on potentially hazardous biological agents?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.