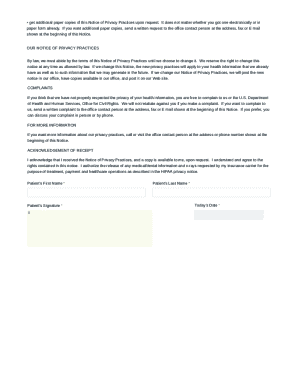
Get the free Authr for Rel Med Rec TO CAS
Get, Create, Make and Sign authr for rel med



How to edit authr for rel med online
Uncompromising security for your PDF editing and eSignature needs
How to fill out authr for rel med

How to fill out authr for rel med
Who needs authr for rel med?
Authr for Rel Med Form: A Comprehensive Guide to Authorship in Medical Research
Understanding the authorship requirement for medical research
Authorship is a critical aspect of medical research as it provides recognition to individuals who have significantly contributed to the research process. In this context, authorship refers to the credit assigned to those involved in writing a manuscript, conducting research, or providing key insights that shape the final work. Establishing clear authorship is not just a matter of recognition; it also delineates responsibilities among co-authors and ensures accountability for the content presented in the research.
One of the foremost reasons establishing clear authorship is essential is to uphold the integrity and quality of research findings. The role each author plays should be well-defined, ensuring that all contributions are acknowledged and protected under copyright laws. This clarity also prevents disputes over authorship, which can be detrimental not only to relationships among researchers but also to the credibility of their work.
Key components of the Rel Med Form
The Rel Med Form, or authorship declaration form, plays a crucial role in documenting the contributions of each author involved in medical research. This form serves as a formal acknowledgment of roles and responsibilities, helping to safeguard the rights of authors while ensuring ethical practices within the research community. The form typically consists of several key sections, each designed to capture essential information about the authors and their contributions.
The first section, Author Identification, collects personal information and institutional affiliations of all contributing authors. This ensures that credit is accurately attributed and provides a means for verification. Following this, the Contribution Details section requires authors to explain their specific roles in the project, which is critical for establishing transparency and accountability. Lastly, the Acknowledgment of Funding Sources is vital, as it prevents conflicts of interest and highlights the support that facilitated the research.
Steps to complete the authorship for Rel Med Form
Completing the Authr for Rel Med Form requires meticulous attention to detail and careful gathering of relevant information. The first important step involves collecting the necessary documentation that supports the claims made in the authorship form. This may include previous manuscripts, IRB approval letters, and notes detailing contributions made by each author. Having this information readily available will facilitate a smoother completion process of the form.
Once all the required information is gathered, the actual filling out of the form can begin. It is important to adhere to any guidelines provided within the form, ensuring each section is accurately completed to avoid errors. Common pitfalls to watch out for include incorrectly stating contributions or failing to include all authors. After the form is filled, it's advisable to review each author's stated contributions carefully. Tools such as collaborative platforms can aid in verifying the accuracy of each person's input, making the review process more efficient.
Navigating common challenges in authorship agreements
Authorship disputes can arise for numerous reasons, such as differing expectations regarding contributions or unclear agreements at the outset of the project. To prevent these disagreements, it’s vital to establish a clear authorship agreement from the beginning of a research project. Regular discussions about roles and contributions help maintain transparency and can resolve potential misunderstandings early on, saving the team from conflict and distress later.
In instances where disputes do arise, there are strategies to effectively manage and resolve them. Engaging an impartial mediator can help facilitate discussion among team members and assist in reaching a consensus. It’s also important to document all decisions made regarding authorship throughout the research process to provide a reference point if conflicts occur. Additionally, misrepresentation issues can arise when authors fail to disclose their true level of contribution. This not only jeopardizes authorship rights but can lead to ethical violations, resulting in potential retractions of published work.
Tools and resources available on pdfFiller
pdfFiller provides an innovative platform designed specifically for completing the Rel Med Form efficiently. One of its main benefits is cloud-based accessibility, allowing researchers to access the form from anywhere, at any time, streamlining the authorship documentation process. This flexibility is essential for teams that may be working under tight timelines or geographical constraints.
Additionally, pdfFiller offers interactive tools for editing and signing documents, ensuring that all contributions are documented accurately and securely. Collaboration features are particularly beneficial; they allow team members to work on documents simultaneously, track changes, and maintain a seamless workflow. Moreover, document tracking and history tools within pdfFiller enable researchers to keep a comprehensive record of edits made to the authorship form, safeguarding the integrity of the revision process.
Tips for ensuring compliance with relevant guidelines
Maintaining compliance with ethical guidelines for authorship is crucial in medical research. Key guidelines, such as those outlined by the International Committee of Medical Journal Editors (ICMJE), stress the significance of substantial contributions to the conception, design, execution, or interpretation of a research project as the basis for authorship. Familiarizing yourself and your co-authors with these guidelines will help safeguard the rights and responsibilities of all contributors.
Many institutions also have specific requirements that must be followed when submitting manuscripts. Understanding these institutional policies can vary significantly and may impact both how authorship is established and how research needs to be reported. Therefore, it's essential for researchers to proactively seek clarity on these institutional directives to ensure compliance and avoid potential conflicts.
Case studies: Successful authorship management
Successful authorship documentation can significantly impact the integrity and credibility of research publications. Numerous case studies exemplify best practices, including transparent authorship agreements and clear delineation of contributions. For instance, a notable publication on clinical trials illustrated the effectiveness of drafting an authorship agreement early in the project, documenting each author’s contributions thoroughly, which streamlined the publication process and fostered collaboration.
Conversely, there are instances where poor management of authorship led to significant conflicts and retractions. A well-documented case involved a multi-disciplinary research project where a lack of clarity on contributions resulted in multiple authors being incorrectly identified. This scenario ultimately led to a retraction and damaged the reputations of those involved. Learning from these real-world scenarios underscores the importance of commitment to transparent and thorough authorship documentation.
Frequently asked questions about authorship for Rel Med Form
As researchers navigate the landscape of authorship continually, inquiries regarding authorship qualification often arise. One common question is: Who qualifies as an author? Typically, anyone who has made substantial contributions to the research process—be it in the study design, methodology, or manuscript preparation—may be considered. It’s essential to recognize that this criterion can vary based on industry standards and institutional guidelines.
Another often-asked question is, 'What should I do if I eliminate or add an author post-submission?' Transparency is crucial in this process. If an author's contributions change before submission, it is essential to communicate these changes promptly to all co-authors and the journal's editorial team to update the authorship accordingly. For further guidance, researchers can consult institutional resources or professional organizations that provide support in these matters.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get authr for rel med?
How do I edit authr for rel med in Chrome?
Can I sign the authr for rel med electronically in Chrome?
What is authr for rel med?
Who is required to file authr for rel med?
How to fill out authr for rel med?
What is the purpose of authr for rel med?
What information must be reported on authr for rel med?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.