Get the free CLIA Brochures - dia-hfd iowa
Get, Create, Make and Sign clia brochures - dia-hfd



How to edit clia brochures - dia-hfd online
Uncompromising security for your PDF editing and eSignature needs
How to fill out clia brochures - dia-hfd

How to fill out clia brochures
Who needs clia brochures?
CLIA brochures - DIA-HFD form: Your Comprehensive How-to Guide
Understanding the CLIA brochures and DIA-HFD form
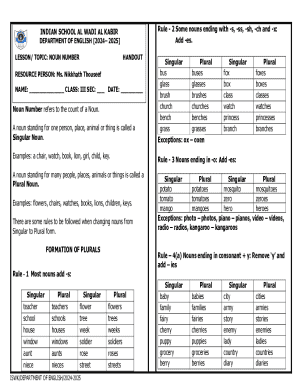
CLIA, or Clinical Laboratory Improvement Amendments, plays a crucial role in ensuring the accuracy and reliability of laboratory testing. These federal regulations set forth quality standards that laboratories must adhere to, thereby safeguarding public health by ensuring that tests are performed correctly and reported accurately. An essential component of CLIA regulations involves the use of various forms and brochures, with a specific focus on the DIA-HFD form which is vital for maintaining compliance in healthcare laboratories.
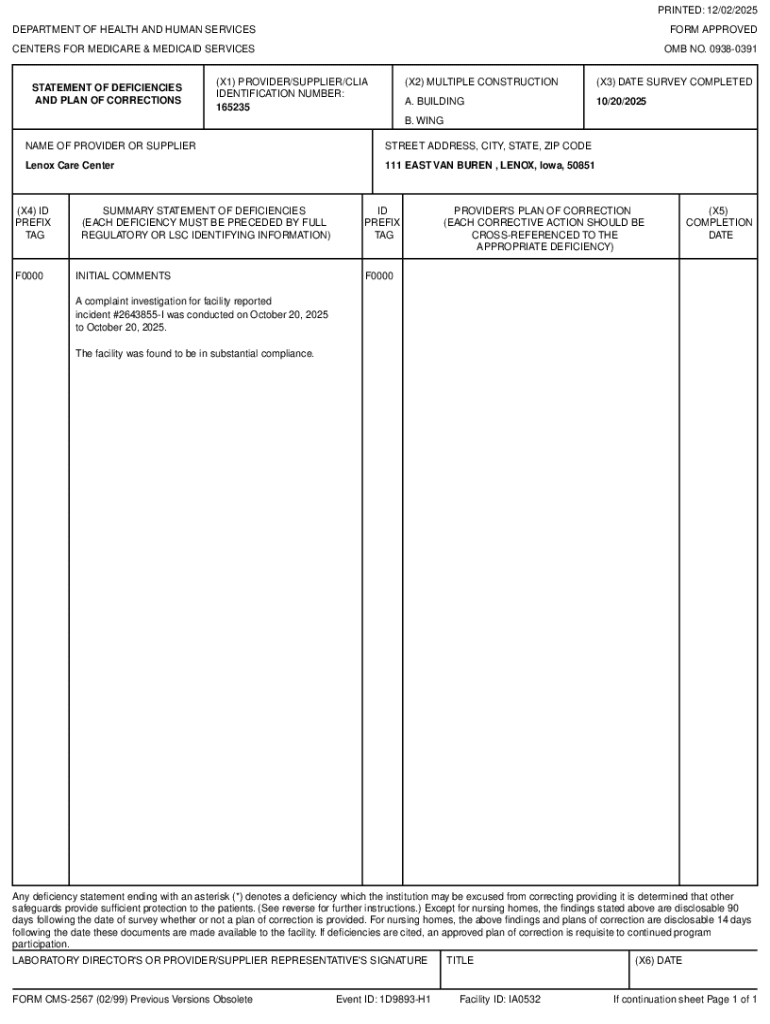
The DIA-HFD form is a specific document that assists laboratories in reporting their hematology and flow cytometry data in accordance with CLIA requirements. This form is crucial for laboratories that perform tests in these areas, ensuring not only compliance but also the high quality of test results that healthcare providers rely upon. Understanding which types of healthcare entities require this form, such as hospitals, independent labs, and blood banks, is instrumental for laboratory administrators.
Accessing the CLIA brochures and DIA-HFD form
Finding the right resources for CLIA compliance begins with the official websites managed by the Centers for Medicare & Medicaid Services (CMS). Users can easily navigate these websites to access a variety of materials, including CLIA brochures and the DIA-HFD form. This official platform not only provides downloadable materials but also offers guidance on navigating compliance effectively.
For a more user-friendly experience, pdfFiller serves as an excellent alternative. Users can access, fill out, and manage these documents online, making the submission process seamless and efficient. To get started, visit the pdfFiller platform, where you can search for the DIA-HFD form directly and access CLIA brochures essential for enhancing your laboratory's operational compliance.
Filling out the DIA-HFD form
Filling out the DIA-HFD form requires attention to detail. The form consists of essential fields including personal and laboratory information, specifications of the tests performed, and a specific section for reporting results. Each of these fields must be completed accurately to avoid discrepancies that could lead to compliance issues. One common error is neglecting to double-check the information entered, which can be mitigated by thorough review before submission.
To provide clear instructions, it's advisable to break down each section methodically. Begin with personal details—such as the name and contact information of the laboratory technician—following up with comprehensive details on the types of tests performed, their specifications, and any relevant results. By systematically addressing each component, the chances of errors decrease, and the quality of submissions improve.
Utilizing pdfFiller for form completion
With pdfFiller, users can enjoy a streamlined experience when completing the DIA-HFD form. Digital tools allow for easy filling, editing, and storing of documents, which significantly reduces the time taken to manage paperwork. Features like auto-fill and collaboration tools enable multiple team members to work on the same document, ensuring all necessary details are captured efficiently.
The benefits of editing directly on pdfFiller are manifold; not only can users save time, but they also mitigate the risks associated with filling out forms incorrectly. Utilizing cloud-based storage allows for real-time updates and easy access, meaning that any necessary changes or adjustments can be made quickly. This dynamic environment helps in tracking the document's progress and facilitates the overall compliance process.
Signing and submitting the DIA-HFD form
The signing process for the DIA-HFD form can now be conducted electronically thanks to features available on pdfFiller. eSigning tools offer a convenient way for users to finalize their documents without the need for printing, scanning, or physical signatures. It is imperative to understand the legal considerations associated with eSigning, ensuring that all signatories fulfill the necessary requirements for validation.
Once signed, the submission of the completed form is the final step in the process. pdfFiller provides clear guidelines on how to submit the completed form electronically, typically via the CMS submission portal or other designated platforms. Establishing awareness of the important deadlines and acceptable submission formats is critical to maintain compliance and the integrity of the laboratory’s operations.
Managing the DIA-HFD form with pdfFiller
After submission, managing the DIR-HFD form becomes essential for tracking compliance and revisiting access records. pdfFiller offers robust tools for tracking submission statuses, permitting users to monitor where their forms stand within the review process. This can alleviate stress and uncertainty following submission, allowing laboratory administrators to focus on other pressing tasks without fear of missing out on crucial follow-ups.
Collaboration among team members also becomes streamlined with pdfFiller. Utilizing shared workspaces allows multiple users to contribute input and suggestions on the submitted form. Version control features are vital for keeping track of changes, ensuring that everyone involved is on the same page regarding the form's updates and revisions.
Frequently asked questions (FAQs)
As you navigate the complexities of the DIA-HFD form, you may encounter questions regarding common issues. Many individuals report uncertainties in filling out certain fields, and a quick review of troubleshooting tips can be beneficial. It's essential to have clear instructions readily available, reducing the likelihood of errors which could delay the compliance process.
In addition, staying informed about updates to CLIA regulations can greatly influence how you approach the DIA-HFD form. Regularly checking reliable sources and being proactive about changes will help maintain compliance. Reach out to pdfFiller's customer support for assistance on form-related queries to clarify any further doubts you may have.
Additional tools and resources
Beyond the DIA-HFD form, several other CLIA forms and brochures may be relevant to your laboratory operations. Being aware of these additional resources can provide comprehensive coverage for compliance and operational efficiency. These documents may offer guidelines on various laboratory practices and help assure the quality of clinical testing.
Moreover, understanding best practices for document management within pdfFiller can lead to improved organizational efficiency. Engaging with further reading on pdfFiller's features enhances not only knowledge but also equips users with skills necessary for optimized document and compliance management.
Tips for effective document management
Optimizing workflow efficiency while using pdfFiller can significantly enhance how forms are handled in your laboratory. Setting up a systematic approach to managing multiple documents, utilizing teams effectively, and ensuring timely revisions ensures that the compliance process remains uninterrupted. Utilizing comment features can assist in obtaining feedback and driving collaboration, which further streamlines operations.
In addition to workflow optimization, maintaining compliance and record-keeping is of utmost importance. This not only safeguards patient data but offers a layer of protection against potential legal challenges. Following best practices such as organizing forms in accessible folders, implementing secure data handling protocols, and routinely checking for updates will contribute to effective document management and compliance adherence.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify clia brochures - dia-hfd without leaving Google Drive?
How can I send clia brochures - dia-hfd for eSignature?
How do I fill out clia brochures - dia-hfd on an Android device?
What is clia brochures?
Who is required to file clia brochures?
How to fill out clia brochures?
What is the purpose of clia brochures?
What information must be reported on clia brochures?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.