GMP SOP TEM-265 2013-2025 free printable template
Show details
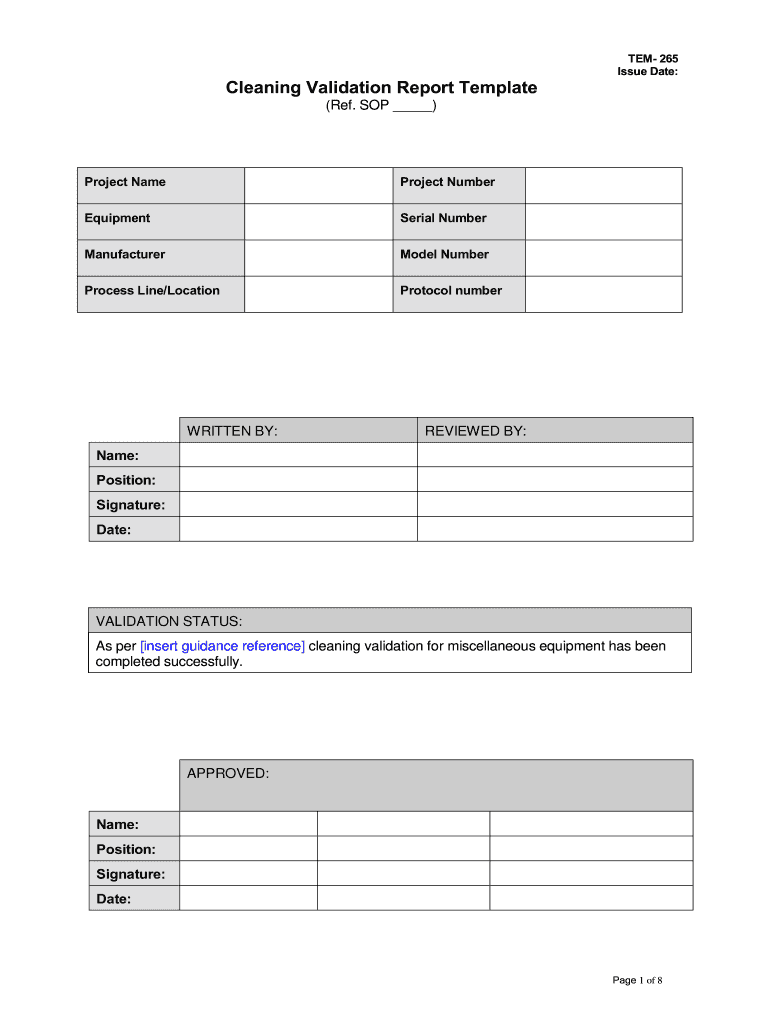
TEM- 265 Issue Date Cleaning Validation Report Template Ref. SOP Project Name Equipment Serial Number Manufacturer Model Number Process Line/Location Protocol number WRITTEN BY REVIEWED BY Name Position Signature Date VALIDATION STATUS As per insert guidance reference cleaning validation for miscellaneous equipment has been completed successfully. APPROVED Page 1 of 8 Microbial Removal* Following cleaning and sanitizing swab samples were taken and tested for microbial levels. All results were...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign validation report template word form
Edit your cleaning validation report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your cleaning validation report pdf form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing cip validation report template online
Follow the guidelines below to use a professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit process validation report format. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out signature cleaning form
How to fill out GMP SOP TEM-265
01
Begin with the title section and ensure it's correctly labeled as GMP SOP TEM-265.
02
Fill in the date of the procedure to keep track of its relevance.
03
Specify the department or team responsible for the SOP.
04
Clearly outline the purpose of the SOP, detailing its objectives.
05
List all relevant definitions and abbreviations to avoid confusion.
06
Detail the step-by-step procedures to be followed in GMP practices.
07
Include any necessary references to regulatory guidelines or standards.
08
Review the document for clarity and completeness.
09
Obtain necessary approvals from management or relevant authorities.
10
Distribute the SOP to all necessary stakeholders and train employees accordingly.
Who needs GMP SOP TEM-265?
01
Employees working in production and quality assurance teams.
02
Regulatory compliance officers looking to ensure adherence to GMP standards.
03
New hires who require training in GMP procedures.
04
Management personnel overseeing GMP operations.
Fill
cleaning validation protocol template
: Try Risk Free
People Also Ask about cleaning validation protocol
What is the conclusion for cleaning validation report?
CONCLUSION: Cleaning validation provides a means of proving that the contamination levels have been reduced below contamination acceptance limits.
What is required for medical device cleaning validation?
A medical device cleaning validation requires that the device is soiled with biological material in a manner that is clinically relevant. The soil will often depend on the type of device being tested, but typically is a mixtures of proteins, hemoglobin, and carbohydrates.
What ISO standard is cleaning validation?
In order to determine the risks of a cleaning process (also at a contract manufacturer) the ISO standard 14971 or the ICH document Q9 are mentioned as supporting documents. The risk analysis should also include activities that also have a cleaning effect, such as passivation or surface treatment.
What is the difference between cleaning validation and cleaning verification?
The one key difference is that each cleaning verification trial or process needs to be sampled and tested to ensure it meets the acceptance criteria. Cleaning verification also allows the cleaning process to be altered each time in order for it to be optimized based on the visual and test results.
What is the 10 ppm rule cleaning validation?
Acceptance Criteria Chemical Criterion: no more than 10 ppm (parts per million) of a product should be detected in another product and/or no more than 0.1% of the normal therapeutic dose of a product should appear in the maximum daily dose of another product.
How do you write a cleaning validation report?
Cleaning Validation Protocol Format – Objective: A brief description of the purpose of the validation study. Scope: This section must include an extent of the cleaning validation protocol. Responsibilities: Training: Record –Training. Protocol signature log: Cleaning Procedure: Sampling Procedure:
What is ISO 17664?
Abstract. ISO 17664:2004 specifies the information to be provided by the medical device manufacturer on the processing of medical devices claimed to be resterilizable, and medical devices intended to be sterilized by the processor.
What is cleaning validation in ICH guidelines?
What is Cleaning Validation? Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product contamination. Cleaning validation should be properly documented to demonstrate Current Good Manufacturing Practice (CGMP) for finished pharmaceuticals.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is cleaning validation report template?
Cleaning validation report templates are documents that provide a structure for recording and summarizing the results of cleaning validation activities. They are used to document theresults of cleaning validation studies, including data on the cleaning process, the cleaning agent, and the results of cleaning validation tests. The reports detail the results of cleaning process studies, including data on the cleaning agent used, the results of cleaning validation tests, and the conclusion of whether the process is validated or not. The report template should also include any recommendations or corrective actions that may be necessary to ensure that the cleaning process meets the desired level of validation.
Who is required to file cleaning validation report template?
Cleaning validation report templates are typically required by regulatory authorities such as the Food and Drug Administration (FDA), Environmental Protection Agency (EPA), and other government and international regulatory bodies, when they review a company's cleaning process. The report should include a list of the cleaning methods used, any tests performed to verify the effectiveness of the cleaning, and the results of those tests.
How to fill out cleaning validation report template?
1. Begin by filling in the header section of the report, which should include the date, the purpose of the report, the name of the individual or team responsible for the validation, and the project or facility name.
2. In the “Scope” section of the report, provide an overview of the area that was validated, including any equipment or areas that were included in the validation process.
3. In the “Methodology” section of the report, provide a detailed description of the methods used to validate the area or equipment, including any sampling and testing techniques employed.
4. In the “Results” section of the report, provide a summary of the results of the validation process, including any areas that did not meet the established standards.
5. In the “Conclusion” section of the report, provide a summary of the findings and a recommendation for any additional steps that may be required to ensure that the area or equipment meets the required standards.
6. Finally, include any other relevant information or documents, such as photographs or diagrams, in the “Attachments” section of the report.
What is the purpose of cleaning validation report template?
A cleaning validation report template is used to document the results of a cleaning validation process. It is used to provide evidence that a product or system has been cleaned to an acceptable level of cleanliness and that it meets all relevant requirements. The template also provides an objective assessment of the cleaning process, including any corrective actions, changes or improvements that may need to be made.
What information must be reported on cleaning validation report template?
1. Summary of the cleaning validation process
2. Description of the cleaning method used
3. Acceptance criteria used for the validation
4. Identification of the product/equipment/materials tested
5. Sampling plan and methodology
6. Results of the testing
7. Conclusions and recommendations
8. Signature of the responsible personnel
How can I send validation template for eSignature?
Once your validation report template is complete, you can securely share it with recipients and gather eSignatures with pdfFiller in just a few clicks. You may transmit a PDF by email, text message, fax, USPS mail, or online notarization directly from your account. Make an account right now and give it a go.
How do I make changes in cip validation report?
The editing procedure is simple with pdfFiller. Open your GMP SOP TEM-265 in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I fill out the GMP SOP TEM-265 form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign GMP SOP TEM-265 and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
What is GMP SOP TEM-265?
GMP SOP TEM-265 is a standard operating procedure (SOP) related to Good Manufacturing Practices (GMP) that outlines specific protocols and requirements for manufacturing processes to ensure quality and compliance in production.
Who is required to file GMP SOP TEM-265?
Entities involved in the manufacturing process of pharmaceutical products, including companies and personnel responsible for production, quality control, and regulatory compliance, are required to file GMP SOP TEM-265.
How to fill out GMP SOP TEM-265?
To fill out GMP SOP TEM-265, individuals must carefully follow the instructions provided in the SOP, ensuring all required fields are completed accurately, including sections on processes, personnel involved, and any relevant observations or notes.
What is the purpose of GMP SOP TEM-265?
The purpose of GMP SOP TEM-265 is to establish a clear framework for maintaining quality standards in manufacturing, ensuring that products meet regulatory requirements, and facilitating traceability and accountability in production processes.
What information must be reported on GMP SOP TEM-265?
Information that must be reported on GMP SOP TEM-265 includes details about the manufacturing process, personnel involved, quality checks performed, deviations from standard procedures, and any corrective actions taken.
Fill out your GMP SOP TEM-265 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

GMP SOP TEM-265 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.



























