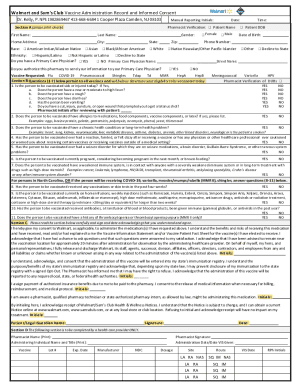
Informed Consent Form For Questionnaire
What is an Informed Consent Form for Questionnaire?
An Informed Consent Form for a questionnaire is a document that participants are required to read and sign before participating in a research study. It outlines the purpose of the study, the procedures involved, any potential risks or benefits, and the rights of the participants. By signing the form, participants indicate that they understand and voluntarily agree to take part in the study.
What are the types of Informed Consent Form for Questionnaire?
There are two main types of Informed Consent Forms for questionnaires:
Explicit Consent Form: This form explicitly states all the key information about the study, including the purpose, procedures, risks, and benefits.
Implied Consent Form: This form is used when the risks associated with the study are minimal and the act of completing the questionnaire implies consent.
How to complete Informed Consent Form for Questionnaire
To complete an Informed Consent Form for a questionnaire, follow these steps:
01
Read the entire form carefully, paying special attention to the purpose of the study and any risks involved.
02
Sign and date the form to indicate your consent to participate in the study.
03
Keep a copy of the signed form for your records.
04
Submit the form to the researcher or organization conducting the study.
pdfFiller empowers users to create, edit, and share documents online. Offering unlimited fillable templates and powerful editing tools, pdfFiller is the only PDF editor users need to get their documents done.
Video Tutorial How to Fill Out Informed consent form for questionnaire
Thousands of positive reviews can’t be wrong
Read more or give pdfFiller a try to experience the benefits for yourself
Questions & answers
How do I write an informed consent form?
Considerations in preparing the informed consent document: Elements of consent present. Complete explanations. Lay language. Protection of confidentiality. No unproven claims of effectiveness. Device studies include a statement that the study includes an evaluation of the safety of the test article.
Does informed consent apply to surveys?
Hard-copy surveys: the cover page should consist of an information letter and a statement that by filling out and returning the survey, the participant gives their informed consent. Participants should be encouraged to tear off the cover page and retain it for their records.
How do you ask for consent in a questionnaire?
How to include a consent question in your survey Include a privacy notice. Make the consent question mandatory. Keep a record of consent.
Why is consent important in questionnaire?
The survey consent form: what to include This ensures everyone understands exactly what's involved in being a respondent. Subsequently, helping to avoid any issues after your data has been collected.
Do you need a consent form for questionnaires?
No- questionnaires are considered self-consenting so it is not necessary for participants to complete a separate consent form. Instead a statement should be included in the introduction to the survey to state that by completing the survey, participants are consenting for their data to be used in the study.
How do you write a consent form for a questionnaire?
Sample consent statement for a survey or questionnaire Thank you very much for agreeing to participate in this survey. The information provided by you in this questionnaire will be used for research purposes. It will not be used in a manner which would allow identification of your individual responses.