Regulate Applicationula Application Gratuit
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

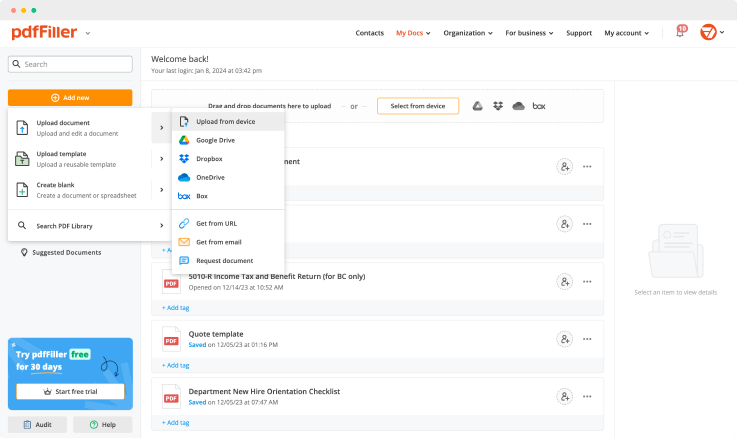
Upload a document

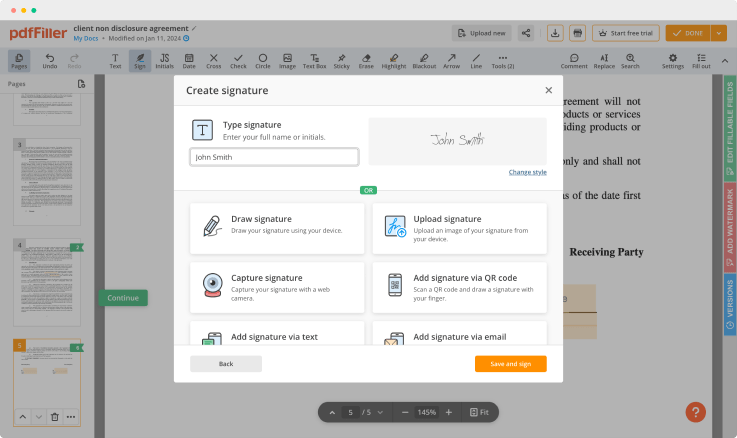
Generate your customized signature

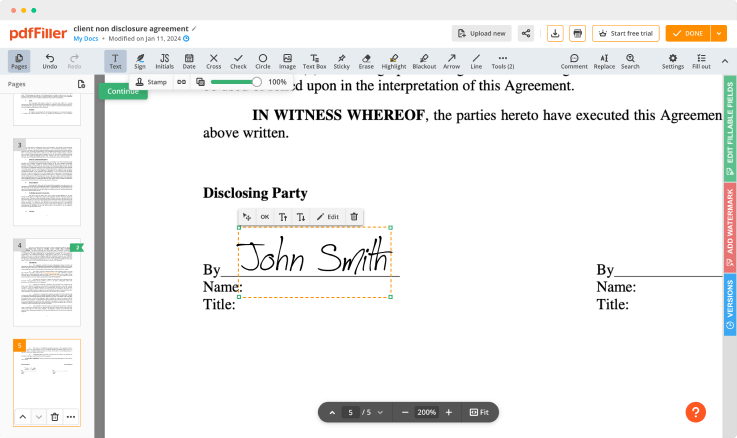
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Regulate Applicationula: Streamline Your Workflow
Regulate Applicationula offers a powerful solution designed to improve your workflow and enhance productivity. With this application feature, you can manage tasks more effectively while ensuring your team stays on track.
Key Features
User-friendly interface that simplifies navigation
Real-time updates to keep everyone informed
Customizable task management tools for unique projects
Integration with popular platforms for seamless operation
Advanced reporting and analytics for data-driven decisions
Potential Use Cases and Benefits
Ideal for project managers seeking to improve team collaboration
Useful for small businesses looking to optimize daily operations
Great for freelancers needing to track multiple clients and deadlines
Beneficial for teams wanting to measure their performance over time
Regulate Applicationula resolves common workflow challenges. By organizing tasks and providing clear visibility, your team can focus on important jobs. This feature lets you save time and reduces the likelihood of missed deadlines. With Regulate Applicationula, you can ensure success in your projects.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What is a regulatory application?
regulatory application. An application made to a health authority by a drug sponsor to investigate, market or license a new product or indication.
What is regulatory filing?
Regulatory Filings means any submission to a Regulatory Authority of any appropriate regulatory application together with any related correspondence and documentation, and will include any submission to a regulatory advisory board, marketing authorization application, and any supplement or amendment thereto.
What SEC filing shows ownership?
The Schedule 13D form not only reveals who own most of the company's shares but also introduces the owner(s) to investors and provides contact information. It's filed within 10 days of any entity acquiring 5% or more of any class of a company's securities.
What are corporate filings?
corporate filing. Any one of a number of forms the Securities and Exchange Commission (SEC) requires every publicly traded company to submit, disclosing its financial condition and material changes in the operation of the organization.
What agency is responsible for ensuring compliance?
OFAC administers and enforces economic and trade sanctions based on U.S. foreign policy and national security goals against targeted foreign states, organizations, and individuals. Compliance in the U.S. generally means compliance with laws and regulations.
What is NDA and IND?
The NDA application is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.S. The data gathered during the animal studies and human clinical trials of an Investigational New Drug (IND) become part of the NDA.
What is the difference between NDA and IND?
Drug Evolution Process of IND, NDA & AND. Drug development is divided into types. If fruitful, this phase is followed by an appeal to the FDA as an Investigational New Drug (IND). After the approval by IND, the next stages are of clinical phases 1, 2, & 3, which require approximately 1-3 years for completion.
Ready to try pdfFiller's? Regulate Applicationula Application Gratuit
Upload a document and create your digital autograph now.