Report Us Phone Object Gratuit
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

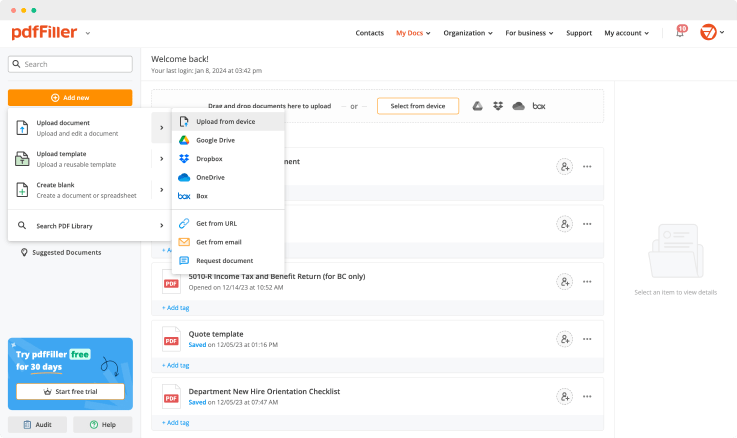
Upload a document

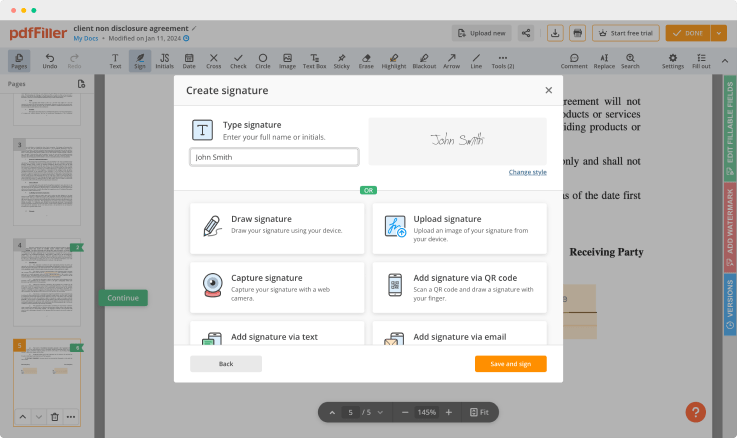
Generate your customized signature

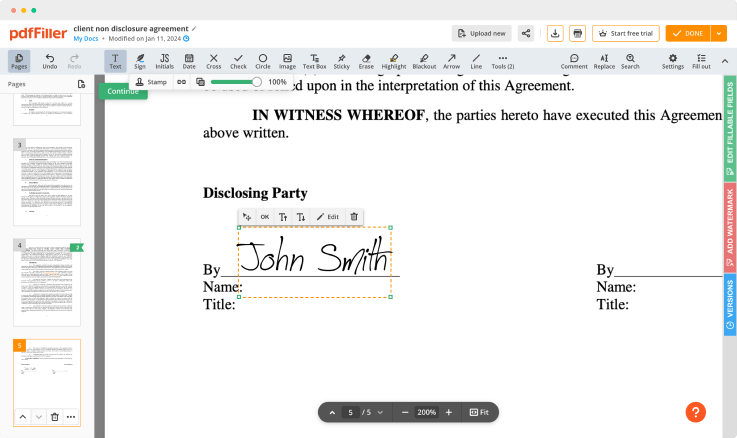
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.
How to Add a Signature to PDF (and Send it Out for Signature)
Watch the video guide to learn more about pdfFiller's online Signature feature

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Report Us Phone Object Feature
Discover the Report Us Phone Object feature, a powerful tool designed to help you streamline communication and enhance your reporting processes. This feature allows users to easily report issues related to phone objects, providing a fast and efficient way to keep your systems running smoothly.
Key Features
Simple reporting interface for quick submissions
Real-time tracking of reported issues
User-friendly notifications for updates
Integration with existing communication systems
Secure handling of sensitive information
Potential Use Cases and Benefits
Business teams can report and resolve phone issues efficiently
IT departments can track and manage phone object performance
Customer service can respond promptly to phone-related concerns
Management can gain insights into recurring phone issues
With the Report Us Phone Object feature, you can tackle communication challenges head-on. By providing a clear and direct way to report problems, you reduce downtime and improve response times. This tool empowers you to ensure that phone systems run effectively, allowing you to focus on what matters most—serving your customers and growing your business.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
How do you report something to the FDA?
Call 1-888-INFO-FDA (1-888-463-6332). Call the FDA Consumer Complaint Coordinator for your state or region.
How do you report an adverse event?
Report Online. Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. Call FDA at 1-800-FDA-1088 to report by telephone. Reporting Form FDA 3500 commonly used by health professionals. View Instructions for Form FDA 3500.
How long do you have to report an adverse event?
Serious and unexpected Ages are expedited by the manufacturer to the FDA within 15 days of the manufacturer's receipt of the ARE. All Ages are also periodically reported by the manufacturer to regulatory agencies through periodic safety reports.
What is an adverse event FDA?
Adverse event means any untoward medical occurrence associated with the use of a drug in humans, whether considered drug related. Life-threatening adverse event or life-threatening suspected adverse reaction.
Why is adverse event reporting important?
The more information generated about specific drugs and devices, the more safe and effective the products can be for the individuals using them. Because the FDA most likely could not handle the influx of information regarding every adverse event, MedWatcher is intended for the reporting of serious adverse events.
What is the FDA MedWatcher program?
MedWatcher is the Food and Drug Administration's Safety Information and Adverse Event Reporting Program. It interacts with the FDA Adverse Event Reporting System (FAIRS or AIRS). MedWatcher is used for reporting an adverse event or sentinel event.
What should I report to MedWatcher?
If you need information or if you have questions or comments about a medical product, please call the FDA's toll-free information line, 1-888-INFO-FDA (1-888-463-6332) Press 2 to report into MedWatcher or for instructions.
What is Form FDA 3500a?
Form FDA 3500A — Mandatory Reporting and Instructions for Completing Form FDA 3500A. Mandatory reporting For use by IND reporters, manufacturers, distributors, importers, user facilities personnel.
Ready to try pdfFiller's? Report Us Phone Object Gratuit
Upload a document and create your digital autograph now.