Get the free Consort 2010 statement: extension to cluster randomised trials - The BMJ
Show details
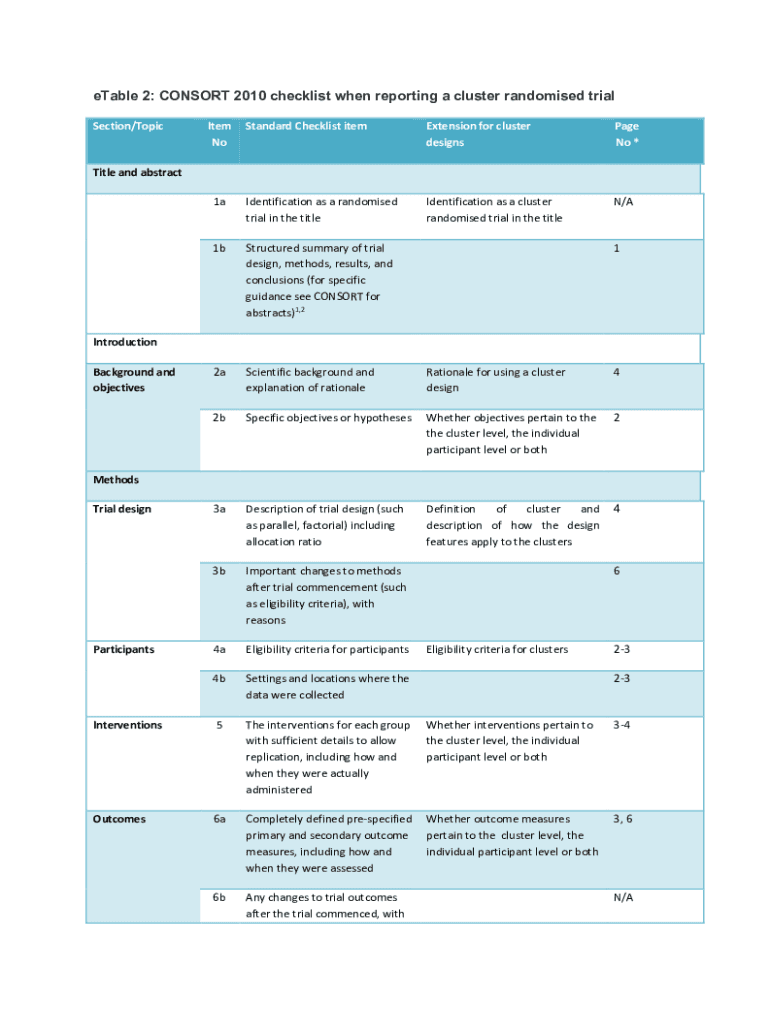
Table 2: CONSORT 2010 checklist when reporting a cluster randomized trial Section/Toxicity Nonstandard Checklist overextension for cluster designs Page No *1aIdentification as a randomized trial in
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign consort 2010 statement extension

Edit your consort 2010 statement extension form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your consort 2010 statement extension form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit consort 2010 statement extension online
Follow the guidelines below to use a professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit consort 2010 statement extension. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out consort 2010 statement extension

How to fill out consort 2010 statement extension
01
To fill out the CONSORT 2010 statement extension, follow these steps:
02
Start with the title section: Provide the title of your study and any other necessary details, such as the authors' names and affiliations.
03
Move on to the abstract section: Summarize the study objectives, methods, results, and conclusions in a concise manner.
04
Fill in the introduction section: Clearly state the background and rationale for the study.
05
Provide details about the trial design and methods, including the participants, interventions, and outcomes.
06
Specify the randomization process, including any blinding procedures.
07
Describe the statistical methods used for data analysis.
08
Present the results section, including both primary and secondary outcomes.
09
Discuss the limitations of the study and any potential biases.
10
Conclude with a summary of the findings and their implications.
11
Finally, provide references to any related publications or studies.
12
Remember to refer to the CONSORT 2010 guidelines for more detailed instructions on each section.
Who needs consort 2010 statement extension?
01
The CONSORT 2010 statement extension is necessary for researchers and authors who are conducting and reporting clinical trials. It helps ensure transparency and standardized reporting of trial protocols and results, facilitating the evaluation and comparison of studies in the field of medicine.
02
Journals, publishers, and reviewers also benefit from CONSORT 2010 statement extension as it enables them to assess the quality and completeness of submitted manuscripts. Adhering to the extension may be required by certain journals or funding agencies for publication or grant consideration. Ultimately, anyone involved in the design, execution, or publication of clinical trials can benefit from the use of CONSORT 2010 statement extension.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my consort 2010 statement extension directly from Gmail?
You can use pdfFiller’s add-on for Gmail in order to modify, fill out, and eSign your consort 2010 statement extension along with other documents right in your inbox. Find pdfFiller for Gmail in Google Workspace Marketplace. Use time you spend on handling your documents and eSignatures for more important things.
How can I send consort 2010 statement extension for eSignature?
When your consort 2010 statement extension is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How do I complete consort 2010 statement extension online?
Completing and signing consort 2010 statement extension online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
What is consort statement extension to?
Consort statement extension is an extension to the CONSORT (Consolidated Standards of Reporting Trials) statement, which provides guidelines for reporting randomized controlled trials.
Who is required to file consort statement extension to?
Researchers and authors conducting randomized controlled trials are required to file consort statement extensions.
How to fill out consort statement extension to?
Consort statement extensions should be filled out following the guidelines provided in the CONSORT statement.
What is the purpose of consort statement extension to?
The purpose of consort statement extension is to improve the transparency and completeness of reporting of randomized controlled trials.
What information must be reported on consort statement extension to?
Consort statement extension should include details on trial methodology, participant recruitment, randomization procedures, and statistical analysis methods.
Fill out your consort 2010 statement extension online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Consort 2010 Statement Extension is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















