Oxygen Electron Configuration
What is oxygen electron configuration?
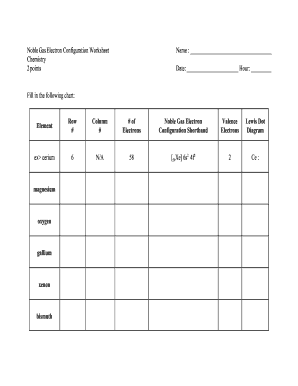
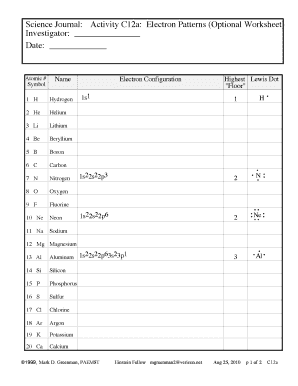
Oxygen electron configuration refers to the arrangement of electrons in the electron shells or energy levels of an oxygen atom. Electrons are arranged in specific orbitals around the nucleus based on their energy levels and the rules of quantum mechanics.
What are the types of oxygen electron configuration?
There are two types of oxygen electron configuration: ground state and excited state. The ground state electron configuration of oxygen is 1s2 2s2 2p4. This means that in its neutral state, oxygen has 8 electrons distributed among the 1s, 2s, and 2p orbitals. However, oxygen can also exist in an excited state with a different electron configuration.
How to complete oxygen electron configuration
To complete the oxygen electron configuration, follow these steps:
Remember to consult a periodic table for reference and to consider the filling order of orbitals based on the Aufbau principle. By following these steps, you can complete the electron configuration of oxygen and understand its distribution of electrons.