Link Table in the Clinical Trial Agreement Template with ease Grátis
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
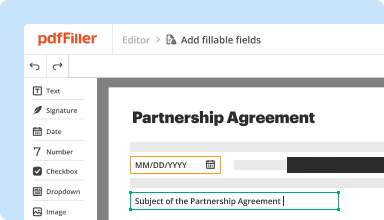
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
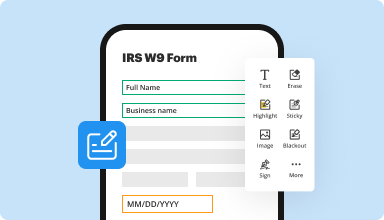
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
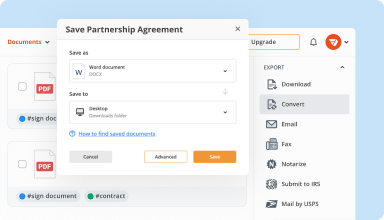
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
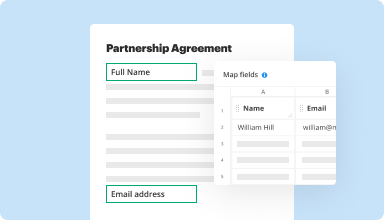
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
difficult to sign and other parties not knowing the software, I feel I need to be cautious. Too costly for a novice one time user. Overall, it has been helpful. thanks!
2015-12-15
Easy and intuitive to use - could fill out forms right away with little effort. Signature capture using the laptop camera did not work - the signature ink lines not clear after cleaning up the image. I scanned my signature at 300dpi using a scanner, and saved that image.
I tried this, liked it, and bought 1 year subscription.
2016-06-07
It popped up along with a State of FL form that I needed to complete. I'm annoyed that they didn't tell me right from the start that you could only use it with a paid prescription.
2017-04-25
eing a new user of this platform I had a major issue and they went over and beyond to fix it and made it up to me even tho it was my fault for not reading. ha
2018-06-26
What do you like best?
Easy to use, saves your signature, can merge
What do you dislike?
The saving document feature is a little slow
What problems are you solving with the product? What benefits have you realized?
Easy to fill PDFs without printing. Can fill as I he info and mAkes edits a breeze
Easy to use, saves your signature, can merge
What do you dislike?
The saving document feature is a little slow
What problems are you solving with the product? What benefits have you realized?
Easy to fill PDFs without printing. Can fill as I he info and mAkes edits a breeze
2019-05-31
This is the greatest website I've found…
This is the greatest website I've found in my 21 professional years. It has saved me so much time and effort I can't believe it. PRICELESS!!!!
2019-11-16
A safe way to fill forms
I wish they had an option for screenshare set up support. Though it took awhile to learn, we are so grateful for what it's allowed our company to do!
LinktoFill allows us to send out one link and receive back filled PDF's instead of uploading over and over and sending back and forth to gather missed info.
The site is hard to learn and takes a bit to get used to.
2019-05-30
5* Customer Service
I am very impressed with the customer service. When I was charged for a years subscription after my free trial, which I queried immediately, I received a full refund within the hour with no quibble.
2021-04-11
Great Customer Service
Used the service on a free trial, was a positive experience but I didn't need it after I was done applying for apartments. Forgot to cancel after my trial and was charged for 2 months, contacted customer service on their live chat and was given the full refund within a minute! World class customer service!
2021-04-07
Link Table Feature in Clinical Trial Agreement Template
The Link Table feature simplifies the management of clinical trial agreements. With this tool, users can easily navigate and connect various elements of their agreements, ensuring clarity and organization throughout the process.
Key Features of the Link Table
Centralized reference for all linked sections
Easy navigation between related documents
Automatic updates when underlying data changes
User-friendly interface for quick access
Use Cases and Benefits
Facilitates better collaboration among team members
Reduces errors and omissions in agreements
Streamlines the review process, saving time
Enhances compliance by tracking related documents
By implementing the Link Table feature, you can tackle common challenges associated with managing clinical trial agreements. It creates a coherent structure that promotes efficiency and accuracy, ultimately leading to smoother project execution. You will find that this tool not only saves time but also helps maintain high standards of compliance and organization.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
Who signs a clinical trial agreement?
Clinical Trial Agreements (CTAs) require signatures from both the trial sponsor and a University institutional official (IO) with signature authority delegated by the Board of Trustees of the Leland Stanford Junior University.
Who are the members of a clinical trial?
Depending on the funding available and complexity of the trial design, the team will usually include: Site Principal Investigator and Associate Investigators. Biostatistician. Study Coordinator/Research Nurse. Data Manager. Programmer. Clinical Trial Pharmacist, for clinical trials of an investigational medicinal product.
Who are the participants of a clinical trial?
Many different types of people take part in clinical trials. Some studies include healthy volunteers, while other studies include patient volunteers. Some studies include both healthy and patient volunteers . In addition, the NHLBI is committed to supporting clinical trials with diverse participants.
Who are the parties involved in a clinical trial?
Participant: A person who volunteers to take part in the clinical trial—such as yourself! Investigator: A researcher who helps conduct the clinical trial—such as a doctor. Study coordinator: A person who works with the investigator to manage the clinical trial—such as a nurse.
Who are the major players in clinical trials?
Who Makes Up a Clinical Research Team? Principal investigator (PI) Also called a primary investigator, this person oversees all aspects of a clinical research study. Study physicians. Research nurse. Study coordinator. Research pharmacists. Participants.
Who are the parties to a clinical trial?
Sponsor: The person or group of people who supervise or fund the trial—such as a drug company. Participant: A person who volunteers to take part in the clinical trial—such as yourself! Investigator: A researcher who helps conduct the clinical trial—such as a doctor.
What is a CTA in a clinical trial?
Definition. A clinical trial agreement (CTA) or clinical study agreement (CSA) is a legally binding agreement that governs the conduct of a particular study and sets forth the obligations of each party to the agreement.
#1 usability according to G2
Try the PDF solution that respects your time.






