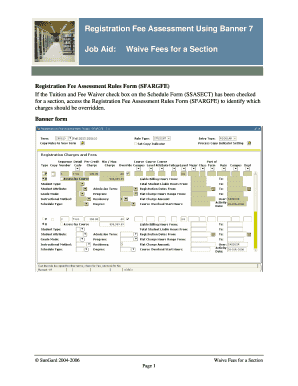
Get the free Structured Product Labeling Validation Procedures for Drug ... - fda
Show details
Structured Product Labeling Release 4 validation procedures v2.0 Structured Product Labeling Validation Procedures for Drug Establishment Registration and Drug Listing Version 2.0 This document describes
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign

Edit your structured product labeling validation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your structured product labeling validation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit structured product labeling validation online
Follow the guidelines below to benefit from a competent PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit structured product labeling validation. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. Sign up for a free account to view.
How to fill out structured product labeling validation

How to fill out structured product labeling validation:
01
Gather all necessary information and documentation about the product being labeled. This includes product specifications, ingredients list, dosing information, and any relevant clinical trial data.
02
Use a specialized software or application that is designed to generate structured product labeling files. These tools provide templates and guidelines to ensure that the labeling is formatted correctly and complies with industry standards.
03
Enter the required information into the appropriate sections of the labeling template. This may include details about the product's indications, contraindications, warnings, precautions, and adverse reactions. It is important to provide accurate and up-to-date information based on the product's current labeling and regulatory requirements.
04
Review and validate the completed structured product labeling file to ensure its accuracy and completeness. This may involve cross-checking the information with reference documents, conducting internal quality checks, and obtaining approval from relevant stakeholders, such as regulatory authorities or product managers.
05
Once the labeling validation process is complete, the structured product labeling file can be submitted to the appropriate regulatory authorities or used for internal purposes within the company. It is important to keep the labeling file updated and promptly make any necessary revisions or updates as required.
Who needs structured product labeling validation:
01
Companies in the pharmaceutical, biotechnology, and medical device industries that manufacture and distribute products that require labeling.
02
Regulatory authorities or agencies responsible for ensuring the safety and efficacy of products in the market.
03
Healthcare professionals who rely on accurate and comprehensive product information to make informed decisions about patient care.
04
Patients and consumers who need access to clear and reliable information about the products they use for their health and well-being.
Fill form : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is structured product labeling validation?
Structured Product Labeling Validation is a process that ensures the accuracy and completeness of structured product labeling documents, which are used to communicate information about healthcare products.
Who is required to file structured product labeling validation?
The entities required to file structured product labeling validation vary depending on the regulatory requirements. Generally, pharmaceutical manufacturers, biotechnology companies, and other stakeholders involved in the production and distribution of healthcare products are required to submit structured product labeling validation.
How to fill out structured product labeling validation?
Structured Product Labeling Validation forms are typically filled out online through a designated platform or software. Users are required to provide accurate and up-to-date information according to the specified guidelines and requirements.
What is the purpose of structured product labeling validation?
The purpose of structured product labeling validation is to ensure that the information provided in the labeling documents of healthcare products is consistent, accurate, and in compliance with regulatory standards. It helps to facilitate the communication of essential product information to healthcare professionals and patients.
What information must be reported on structured product labeling validation?
The information reported on structured product labeling validation includes product identification details, manufacturing information, dosage and administration guidelines, safety and efficacy data, and other relevant information specified by the regulatory authorities.
When is the deadline to file structured product labeling validation in 2023?
The specific deadline to file structured product labeling validation in 2023 may vary depending on the regulatory requirements and jurisdiction. It is recommended to refer to the respective regulatory authorities or consult legal experts for accurate and up-to-date information.
What is the penalty for the late filing of structured product labeling validation?
The penalty for the late filing of structured product labeling validation is typically determined by the regulatory authorities. It may vary based on the jurisdiction and severity of the delay. Penalties can include fines, loss of market authorization, and other regulatory actions.
How can I edit structured product labeling validation on a smartphone?
The easiest way to edit documents on a mobile device is using pdfFiller’s mobile-native apps for iOS and Android. You can download those from the Apple Store and Google Play, respectively. You can learn more about the apps here. Install and log in to the application to start editing structured product labeling validation.
Can I edit structured product labeling validation on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share structured product labeling validation from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
How do I fill out structured product labeling validation on an Android device?
Complete your structured product labeling validation and other papers on your Android device by using the pdfFiller mobile app. The program includes all of the necessary document management tools, such as editing content, eSigning, annotating, sharing files, and so on. You will be able to view your papers at any time as long as you have an internet connection.
Fill out your structured product labeling validation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Not the form you were looking for?
Keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.