This paper is not intended to serve either as a regulatory, law enforcement, or medical professional certification document. The purpose of presenting this paper is to raise awareness that clinical trials have an important function and are not necessarily inherently flawed. We hope that this paper will raise awareness among both investigators and participants that clinical trials for human participants are designed and executed well, with careful evaluation being employed.
The purpose of this document is to highlight the following considerations in the planning, design or execution of any clinical trial, and provide suggestions for how clinical trial participants who are concerned about possible flaws in the design and execution of clinical trials for human participants can contribute to the development of the scientific foundation of this field:
1. Determining that an investigator's methods, methods, methods, methods are ethical (i.e., in line with the ethical conduct of clinical research or clinical trials) and acceptable (i.e., in light of principles, laws and regulations of the relevant discipline or the jurisdiction in which the investigator conducts the study)
2. Finding flaws in a clinical trial design or the design of a clinical trial (i.e., if you are having an issue with the design or execution of a clinical trial for human participants, you should write to the investigator asking for the reasons for your opinion, explaining how you feel these flaws affect your role in the trial. In general, a participant who does not know the details of the design or execution of a study is less likely to be able to evaluate it critically.
3. Knowing about the issues with a clinical trial for human participants to raise questions about the adequacy of the conduct of a particular clinical trial. For example, do I think a specific trial does not comply with regulations in the jurisdiction in which it is being conducted? Do you think a specific trial does not comply with principles of medical ethics or ethical conduct of clinical research?
4. If it is established that a study does not comply with principles of medical ethics or ethical conduct of clinical research, this should prompt a reconsideration of the ethics of conducting that particular trial.
A1.1.

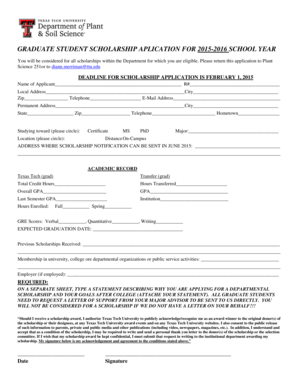
Get the free General Information A0 A0 A0 A0 A0 A0 A0 A0 A0 A0 Respondent Information (Not for Pu...
Show details
Common Data Set 2009-10 A. General Information A0 A0 A0 A0 A0 A0 A0 A0 A0 A0 Respondent Information (Not for Publication) Name: JR Berlin Title: Institutional Research Coordinator Office: Institutional
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign

Edit your general information a0 a0 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your general information a0 a0 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit general information a0 a0 online
To use the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit general information a0 a0. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Fill form : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is general information a0 a0?
General information a0 a0 refers to...
Who is required to file general information a0 a0?
The individuals or businesses required to file general information a0 a0 are...
How to fill out general information a0 a0?
To fill out general information a0 a0, you need to...
What is the purpose of general information a0 a0?
The purpose of general information a0 a0 is...
What information must be reported on general information a0 a0?
The information that must be reported on general information a0 a0 includes...
When is the deadline to file general information a0 a0 in 2023?
The deadline to file general information a0 a0 in 2023 is...
What is the penalty for the late filing of general information a0 a0?
The penalty for the late filing of general information a0 a0 is...
Can I create an electronic signature for the general information a0 a0 in Chrome?
You certainly can. You get not just a feature-rich PDF editor and fillable form builder with pdfFiller, but also a robust e-signature solution that you can add right to your Chrome browser. You may use our addon to produce a legally enforceable eSignature by typing, sketching, or photographing your signature with your webcam. Choose your preferred method and eSign your general information a0 a0 in minutes.
Can I create an electronic signature for signing my general information a0 a0 in Gmail?
Use pdfFiller's Gmail add-on to upload, type, or draw a signature. Your general information a0 a0 and other papers may be signed using pdfFiller. Register for a free account to preserve signed papers and signatures.
How do I fill out general information a0 a0 on an Android device?
Use the pdfFiller app for Android to finish your general information a0 a0. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
Fill out your general information a0 a0 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Not the form you were looking for?
Keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.