Get the free NIDCR Serious Adverse Event Form. Form for reporting SAE - hyposalivation
Show details
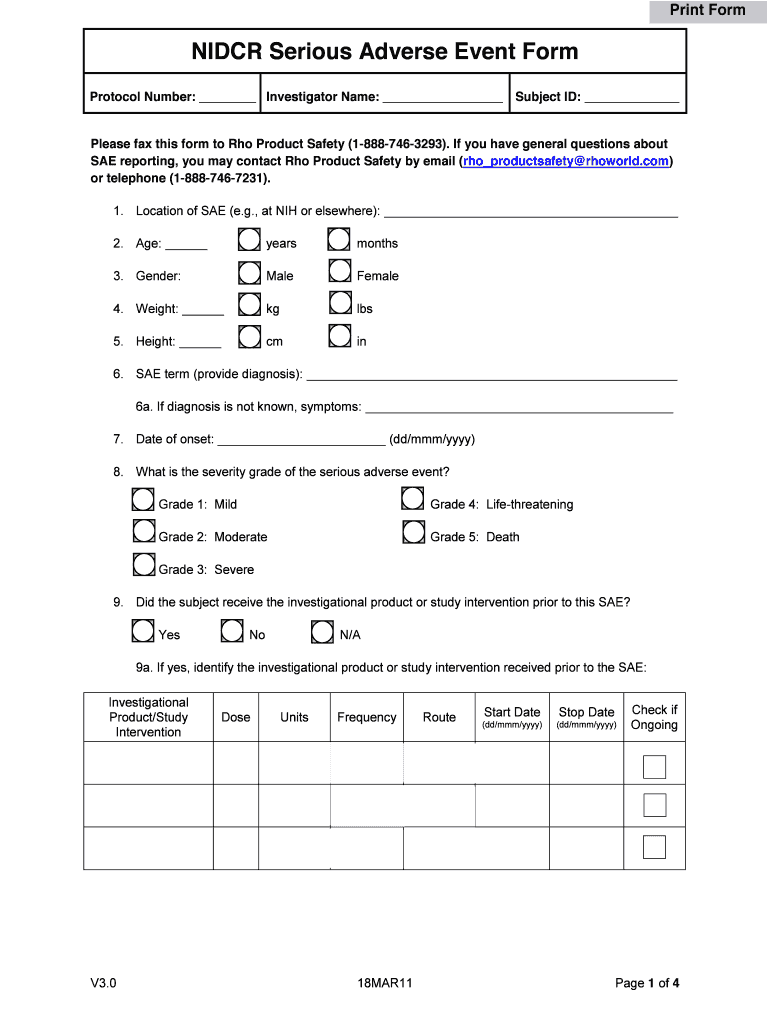
Print Form NI DCR Serious Adverse Event Form Protocol Number: Investigator Name: Subject ID: Please fax this form to Rho Product Safety (18887463293×. If you have general questions about SAE reporting,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign

Edit your nidcr serious adverse event form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your nidcr serious adverse event form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit nidcr serious adverse event online
To use the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit nidcr serious adverse event. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward. Try it now!
How to fill out nidcr serious adverse event

How to fill out NIDCR serious adverse event:
01
Determine the type of serious adverse event (SAE) you are reporting. NIDCR requires the reporting of any adverse event that results in death, a life-threatening situation, hospitalization or prolongation of existing hospitalization, persistent or significant disability, or a congenital anomaly/birth defect.
02
Collect all relevant information about the SAE. This includes the participant's demographic information, the nature and severity of the event, the date and time of onset, any medical interventions or treatments provided, and any other pertinent details.
03
Complete the NIDCR Serious Adverse Event form. This form can usually be found on the NIDCR website or obtained from your institution's research compliance office. Fill in all required fields accurately and legibly.
04
Attach any supporting documentation. If there are medical records, laboratory reports, or other documents that provide additional information about the SAE, make sure to include copies with the completed form.
05
Submit the completed form and supporting documents to the appropriate authorities as specified by your institution's research compliance guidelines. This may involve submitting it to the local Institutional Review Board (IRB), the NIDCR, or other designated entities.
Who needs NIDCR serious adverse event?
01
Researchers conducting clinical trials or studies that are funded or supported by the National Institute for Dental and Craniofacial Research (NIDCR) need to report serious adverse events.
02
The NIDCR requires that both principal investigators and their study teams be responsible for identifying and reporting any serious adverse events that occur during the course of their research.
03
Institutional Review Boards (IRBs) overseeing NIDCR-funded studies also play a crucial role in ensuring that serious adverse events are properly reported and managed. They review the reported events and assess their impact on participant safety and the overall study conduct.
Fill form : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is nidcr serious adverse event?
NIDCR serious adverse event is an adverse event that is unexpected, serious, and possibly related to the use of a NIDCR product or intervention.
Who is required to file nidcr serious adverse event?
Researchers, sponsors, and investigators conducting NIDCR-funded clinical trials are required to file NIDCR serious adverse event reports.
How to fill out nidcr serious adverse event?
NIDCR serious adverse event reports can be filled out using the FDA Form 3500A or through the NIDCR Adverse Event Reporting System (NAERS).
What is the purpose of nidcr serious adverse event?
The purpose of NIDCR serious adverse event reporting is to monitor the safety of NIDCR products and interventions during clinical trials and to ensure the protection of human subjects.
What information must be reported on nidcr serious adverse event?
NIDCR serious adverse event reports must include information about the adverse event, the NIDCR product or intervention involved, the subject's medical history, and the investigator's assessment of causality.
When is the deadline to file nidcr serious adverse event in 2024?
The deadline to file NIDCR serious adverse event reports in 2024 is within 7 calendar days of becoming aware of the event.
What is the penalty for the late filing of nidcr serious adverse event?
The penalty for late filing of NIDCR serious adverse event reports may include warnings, suspension of clinical trials, or enforcement actions by regulatory authorities.
How can I modify nidcr serious adverse event without leaving Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including nidcr serious adverse event, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How do I make edits in nidcr serious adverse event without leaving Chrome?
Install the pdfFiller Google Chrome Extension to edit nidcr serious adverse event and other documents straight from Google search results. When reading documents in Chrome, you may edit them. Create fillable PDFs and update existing PDFs using pdfFiller.
Can I sign the nidcr serious adverse event electronically in Chrome?
Yes. With pdfFiller for Chrome, you can eSign documents and utilize the PDF editor all in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a handwritten signature image. You may eSign your nidcr serious adverse event in seconds.
Fill out your nidcr serious adverse event online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Not the form you were looking for?
Keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.





















