Clinical trial agreement Sample online for Free
Create your document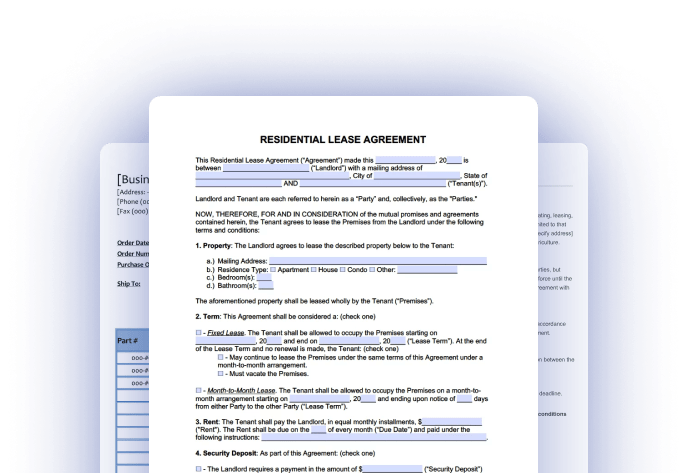
Every progressive person is searching for a solution that will eliminate papers and speed up the document workflow. PDFfiller provides a wide variety of multifunctional tools that help to fill Clinical trial agreement electronically. Any PDF template, form, contract or letter can be edited digitally without changing its format. The powerful toolkit consists of the set of instruments that provide every customer with the opportunity to add textual data, images, sticky notes, watermarks and sign the necessary sample. In addition to that, text can be bold, italic, underlined, highlighted, erased and removed right from PDFs. Try more features and enjoy digital independence with PDFfiller!
The document should answer these basic questions
Who clinical trial agreement checklist?
Where clinical trial agreement checklist?
When clinical trial agreement checklist?
What does clinical trial agreement mean?
How to negotiate clinical trial agreements?
Why clinical trial agreement checklist?
Other names for the document:
Model clinical trial agreement
Clinical trial agreement checklist
Model clinical trial agreement 2018
Types of clinical trial agreements
Clinical trial agreement template india
Master clinical trial agreement template
Clinical trial agreement fda
Indemnification in clinical trial agreements
Other ready to use document templates
Co-branding agreement Sample online for Free
Every progressive person is searching for a solution that will eliminate papers ...
Learn more
Codicil to will Sample online for Free
Every progressive person is searching for a solution that will eliminate papers ...
Learn more
Cohabitation agreement Sample online for Free
Every progressive person is searching for a solution that will eliminate papers ...
Learn more
pdfFiller scores top ratings in multiple categories on G2
All-in-one PDF software
A single pill for all your PDF headaches. Edit, fill out, eSign, and share – on any device.
How to create a Clinical trial agreement Sample online for Free
01
To start, click
CREATE YOUR DOCUMENT. This will take you directly to pdfFiller’s advanced editor.
02
Choose an industry-specific sample or build your own from scratch.
03
Use the advanced editor to get your document exactly how you need it; type text, adjust its size, font, and style, highlight, add bullet points, tables, images, hyperlinks, and more.
04
Build a professional document by adding smart fillable fields. Select the fields you need from the panel on the right and drag & drop them anywhere you need on the page.
05
Once you have finished building your document, click
Done to save the changes.
06
Send the document for review or signing by emailing it or generating a public link. In addition, you have the option to download it or print it out.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Lisa F
2014-06-05
Really good although if you had some type of conversion to excel function, that would be awesome. Thank you.

Kristen Bailey
2019-01-28
What do you like best?
I like how easy it is to use, and how much guidance it offers on how to save and find your documents once you're done. You can easily control where you want your filled information to go, and your documents come out looking professional and polished.
What do you dislike?
There is nothing so far that I dislike. It's very easy to use.
Recommendations to others considering the product:
It's easy and you can learn how to be successful with it instantly, and get right to work.
What problems are you solving with the product? What benefits have you realized?
I receive documents all the time that need to be filled out and without full Adobe Acrobat, they were impossible to work on until I got PDFfiller.
I like how easy it is to use, and how much guidance it offers on how to save and find your documents once you're done. You can easily control where you want your filled information to go, and your documents come out looking professional and polished.
What do you dislike?
There is nothing so far that I dislike. It's very easy to use.
Recommendations to others considering the product:
It's easy and you can learn how to be successful with it instantly, and get right to work.
What problems are you solving with the product? What benefits have you realized?
I receive documents all the time that need to be filled out and without full Adobe Acrobat, they were impossible to work on until I got PDFfiller.

For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What are the three phases of a clinical trial?
Phase 0. Phase 0 trials are the first clinical trials done among people.
Phase I. Phase I trials aim to find the best dose of a new drug with the fewest side effects.
Phase II. Phase II trials further assess safety as well as if a drug works.
Phase III.
Phase IV.
What is a CTA clinical trial application?
A Clinical Trials Application (CTA) is the application/submission to the competent National. Regulatory Authority(ies) for authorization to conduct a clinical trial in a specific country.
What is an example of a clinical trial?
For example, a clinical trial could involve new drugs, medical devices, biologicals, vaccines, surgical and other medical treatments and procedures. Psycho-therapeutic and behavioural therapies help service changes, preventative care strategies and educational interventions are also examples of clinical trials.
What does a CRA do?
A clinical research associate (CRA) is a health care or life sciences professional who oversees clinical trials on behalf of pharmaceutical companies, medical research institutes and government agencies. They are sometimes called clinical monitors or trial monitors.










