How To Find Electronic Configuration Of Elements
What is how to find electronic configuration of elements?
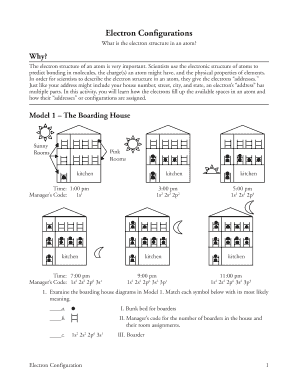
Electronic configuration refers to the arrangement of electrons in an atom or molecule. It determines the chemical properties of an element and plays a crucial role in understanding its behavior in chemical reactions. To find the electronic configuration of an element, you need to follow a specific set of rules and principles.
What are the types of how to find electronic configuration of elements?
There are two commonly used methods to find the electronic configuration of elements: the Aufbau principle and the Pauli exclusion principle. The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy. The Pauli exclusion principle specifies that each orbital can hold a maximum of two electrons with opposite spins. By applying these principles, you can determine the electronic configuration of any element.
How to complete how to find electronic configuration of elements
To find the electronic configuration of an element, follow these steps:
By following these steps, you can accurately determine the electronic configuration of any element in a systematic manner.