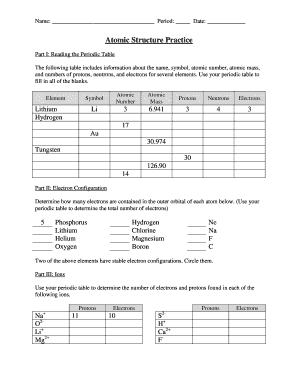
Periodic Table With Atomic Mass
What is periodic table with atomic mass?
The periodic table with atomic mass is a tabular arrangement of chemical elements, organized based on their atomic numbers and atomic masses. It provides valuable information about each element, including its symbol, atomic number, atomic mass, and electron configuration. The atomic mass represents the average mass of an atom of that element, taking into account the different isotopes and their abundance.
What are the types of periodic table with atomic mass?
There are two main types of periodic tables with atomic mass: relative atomic mass and standard atomic weight. The relative atomic mass is the average weighted mass of an element's isotopes relative to the mass of an atom of carbon-12, which is assigned a mass of exactly 12. The standard atomic weight, on the other hand, is the average weighted mass of an element's isotopes taking into account their natural abundance on Earth. Both types provide useful information for understanding the atomic properties of elements.
How to complete periodic table with atomic mass
Completing a periodic table with atomic mass involves several steps:
By following these steps, you can create a comprehensive periodic table that provides valuable insights into the atomic properties of elements. Remember, pdfFiller is a powerful tool that can assist you in creating, editing, and sharing your documents online, including periodic tables with atomic mass.