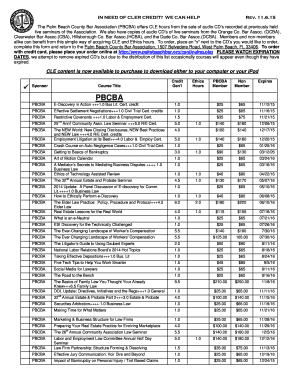
Electron Configuration Chart
What is Electron Configuration Chart?
An Electron Configuration Chart is a graphical representation used to show the arrangement of electrons in an atom or ion. It provides a systematic way of identifying the energy levels and orbital locations of electrons in an atom.
What are the types of Electron Configuration Chart?
There are two types of Electron Configuration Chart: 1. Condensed Electron Configuration: This type of configuration uses noble gas shorthand to represent the electron arrangement in an atom. It starts with the symbol of the noble gas that precedes the given element in the periodic table and then continues with the remaining electron arrangement. 2. Complete Electron Configuration: This type of configuration represents the electron arrangement of an atom without using noble gas shorthand. It includes the detailed distribution of electrons in each energy level and orbital.
How to complete Electron Configuration Chart?
Completing an Electron Configuration Chart involves the following steps: 1. Determine the atomic number of the element for which you want to complete the configuration. 2. Determine the total number of electrons based on the atomic number. 3. Determine the electron arrangement by filling the orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle. 4. Use the given periodic table to determine the number of electrons in each energy level and orbital.
pdfFiller empowers users to create, edit, and share documents online. Offering unlimited fillable templates and powerful editing tools, pdfFiller is the only PDF editor users need to get their documents done.