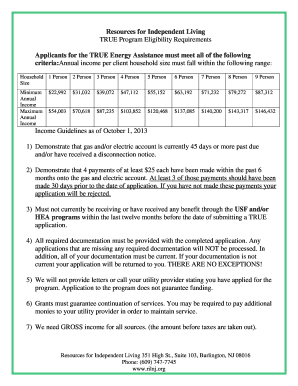
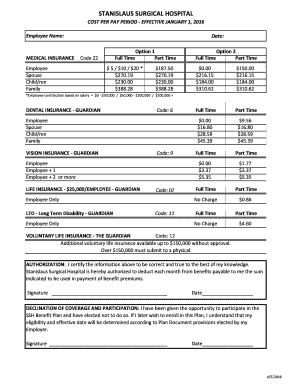
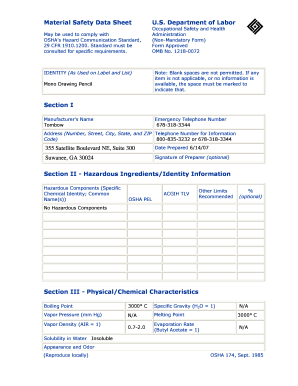
What is a soluble or insoluble chart?
A soluble or insoluble chart is a tool used to determine whether a substance is soluble (able to dissolve) or insoluble (unable to dissolve) in another substance. It provides a visual representation of the solubility of different substances, making it easier to understand and compare their chemical properties.
What are the types of soluble or insoluble chart?
There are two main types of soluble or insoluble charts:
Solubility Chart: This type of chart provides information on the solubility of various substances in a specific solvent. It usually categorizes substances into soluble, insoluble, or partially soluble based on their ability to dissolve in the solvent.
Insolubility Chart: This type of chart focuses on substances that are insoluble in a specific solvent. It helps identify substances that do not dissolve or have low solubility in the chosen solvent.
Mixed Solubility Chart: Some charts combine both soluble and insoluble substances to provide a comprehensive overview of their solubility properties.
How to complete a soluble or insoluble chart
Completing a soluble or insoluble chart is a straightforward process. Here are the steps to follow:
01
Choose a solvent: Select the solvent in which you want to test the solubility of different substances. Common solvents include water, ethanol, and acetone.
02
Gather substances: Collect a variety of substances that you want to test for solubility. These can include salts, sugar, gases, and other organic or inorganic compounds.
03
Conduct tests: Take a small amount of each substance and add it to a separate container of the chosen solvent. Observe whether the substance dissolves completely, partially, or remains insoluble.
04
Record the observations: Document the results of each test in the soluble or insoluble chart. Use the appropriate category (soluble, insoluble, or partially soluble) to categorize each substance based on its solubility in the selected solvent.
05
Analyze the data: Once you have completed the tests and recorded the observations, analyze the data to identify any patterns or trends in the solubility of different substances.
06
Draw conclusions: Based on the data analysis, draw conclusions about the solubility properties of the tested substances in the chosen solvent.
07
Update the chart: Make any necessary updates to the soluble or insoluble chart based on the conclusions drawn from the data analysis.
pdfFiller is an excellent tool that empowers users to create, edit, and share documents online. With its vast collection of fillable templates and powerful editing tools, pdfFiller provides everything you need to efficiently complete your documents. Whether you are editing PDFs, creating forms, or collaborating with others, pdfFiller is the ultimate PDF editor for all your document needs.