Consent Countersign Request For Free
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent

Upload your document to the PDF editor

Type anywhere or sign your form

Print, email, fax, or export

Try it right now! Edit pdf
Users trust to manage documents on pdfFiller platform
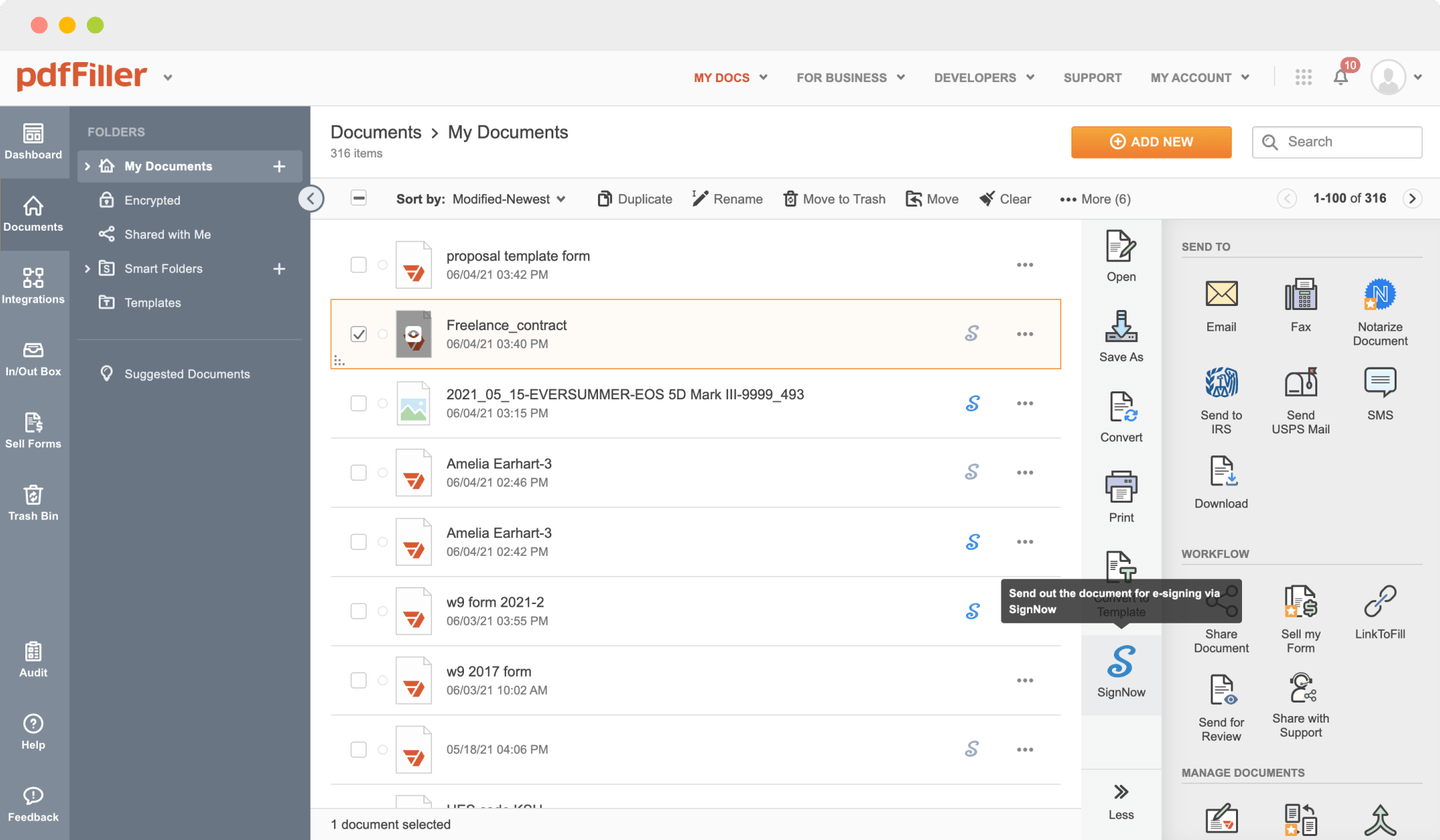
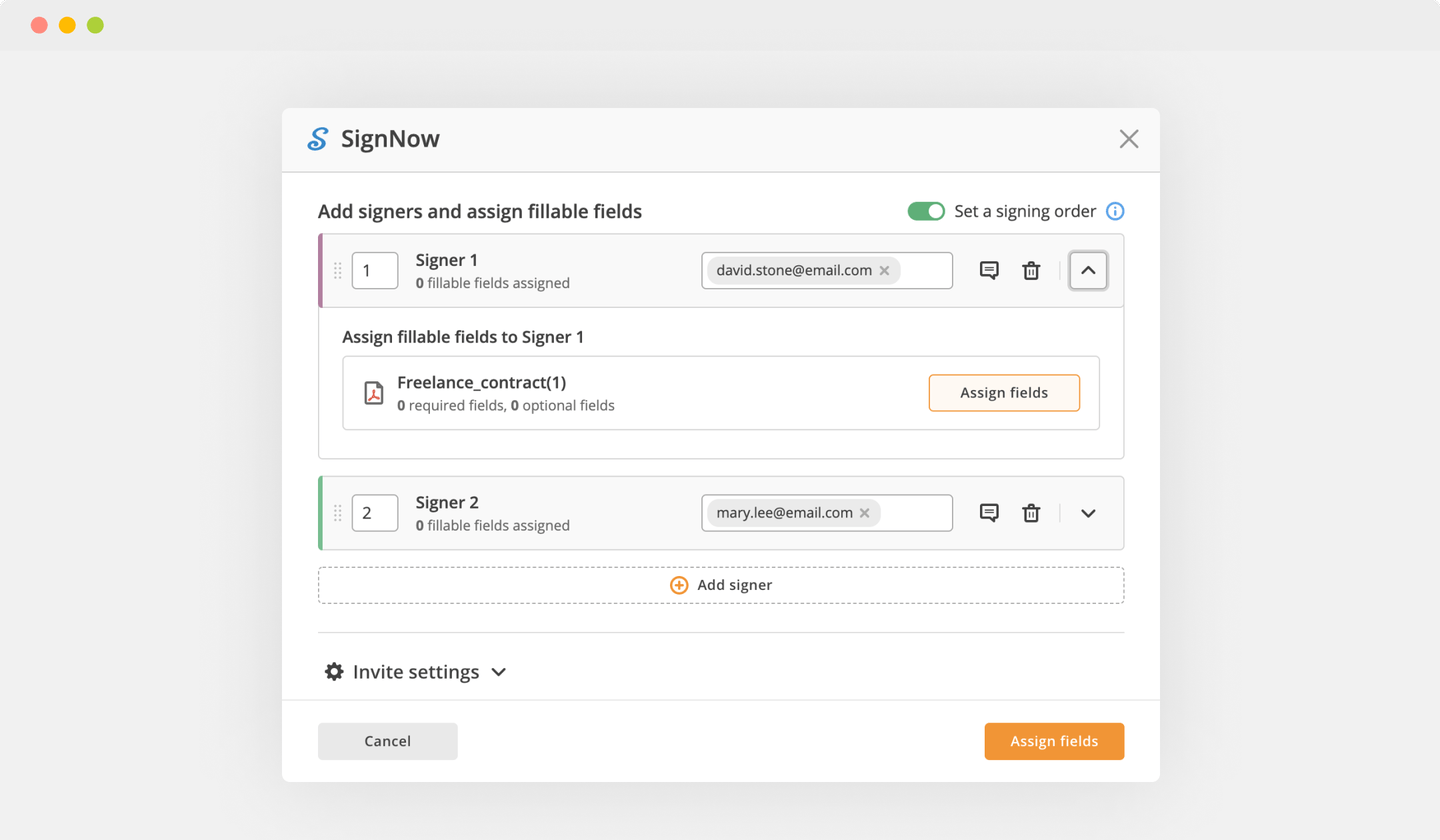
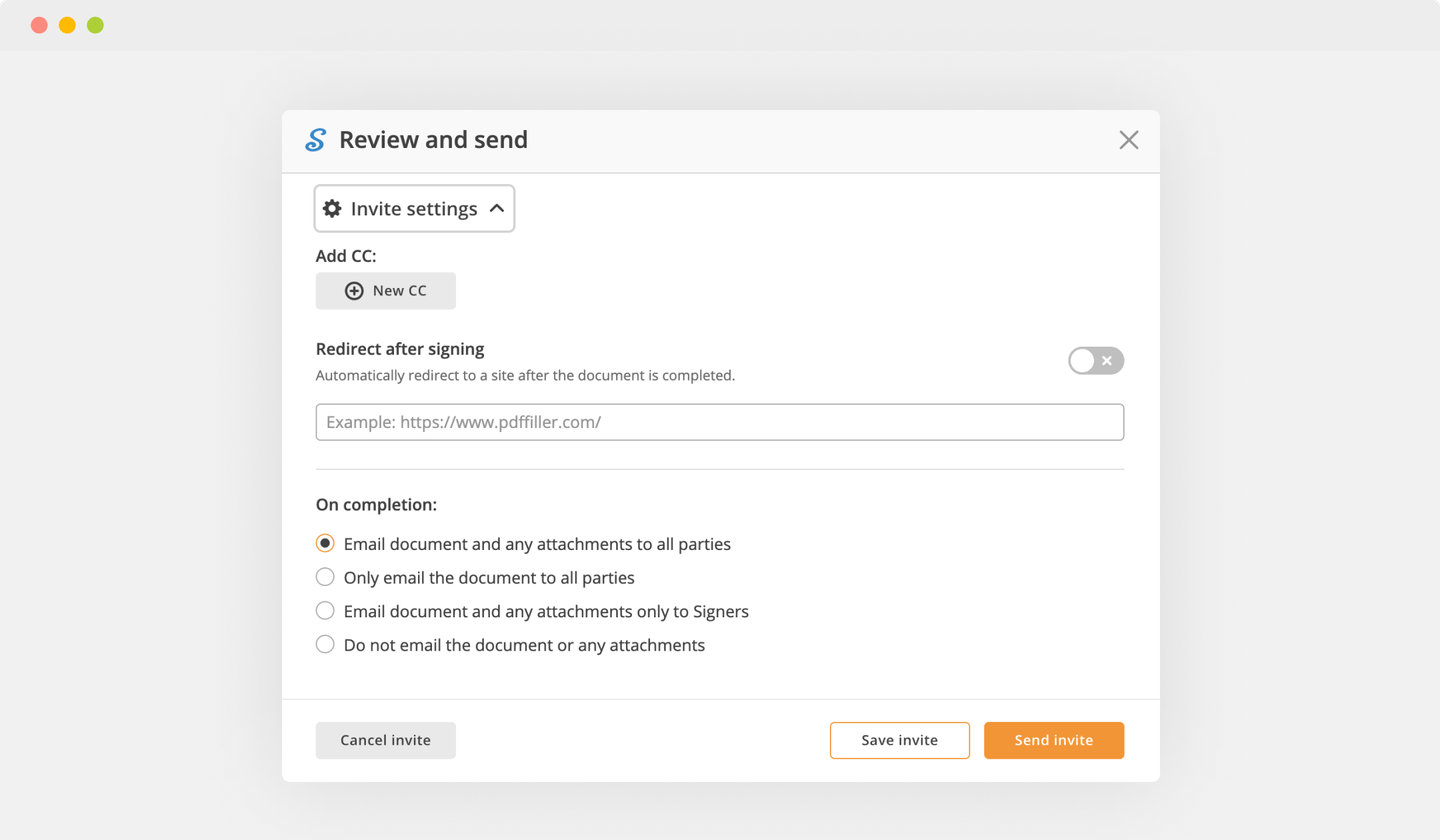
Send documents for eSignature with signNow
Create role-based eSignature workflows without leaving your pdfFiller account — no need to install additional software. Edit your PDF and collect legally-binding signatures anytime and anywhere with signNow’s fully-integrated eSignature solution.
All-in-one PDF software
A single pill for all your PDF headaches. Edit, fill out, eSign, and share – on any device.
pdfFiller scores top ratings in multiple categories on G2
How to Consent Countersign Request
Stuck working with multiple programs to manage documents? Use our all-in-one solution instead. Document management is simpler, fast and efficient using our platform. Create document templates from scratch, modify existing forms, integrate cloud services and other useful features without leaving your account. You can Consent Countersign Request right away, all features are available instantly. Pay as for a basic app, get the features as of pro document management tools. The key is flexibility, usability and customer satisfaction.
How-to Guide
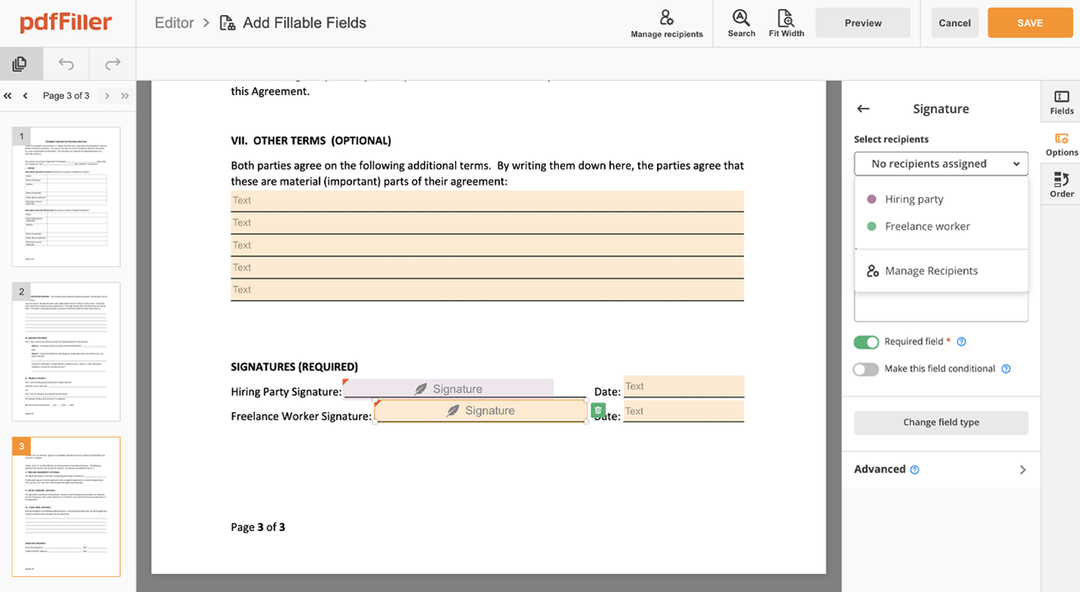
How to edit a PDF document using the pdfFiller editor:
01
Drag and drop your template to the uploading pane on the top of the page
02
Choose the Consent Countersign Request feature in the editor's menu
03
Make the necessary edits to your file
04
Click the “Done" button to the top right corner
05
Rename the document if required
06
Print, save or email the form to your device
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Anonymous Customer
2017-01-20
This tool is AMAZING!!! I've wasted so much time over the years bringing images into Word and overlaying text boxes to accomplish what this tool does SO easily. Thank You! One suggestion: when I click on a document page on the left side of the screen, it would be great if that page opened roughly where I clicked. That is, if I click on the bottom of the page image on the left, it would be great if the page opened with the bottom showing, rather than having to scroll down to the bottom. This would make it the same as Acrobat.

Leonard S
2019-03-09
works, got my document, little squirrely on fill in but I figured it. It takes about 75% more time to fill in, not a seamless experience. If the typing could be done with out having to locate start character, would hlep


Get a powerful PDF editor for your Mac or Windows PC
Install the desktop app to quickly edit PDFs, create fillable forms, and securely store your documents in the cloud.

Edit and manage PDFs from anywhere using your iOS or Android device
Install our mobile app and edit PDFs using an award-winning toolkit wherever you go.

Get a PDF editor in your Google Chrome browser
Install the pdfFiller extension for Google Chrome to fill out and edit PDFs straight from search results.
List of extra features
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Who can witness informed consent?
First and foremost, it is the healthcare practitioner who is performing the procedure's duty to obtain the informed consent of the patient. When a witness signed his or her name on the consent form, the person is attesting to the fact that they saw the person sign the consent form only.
What is the nurses' role in informed consent?
Participating in Obtaining Informed Consent The nurse is responsible and accountable for the verification of and witnessing that the patient or the legal representative has signed the consent document in their presence and that the patient, or the legal representative, is of legal age and competent to provide consent.
Does a physician have to sign an informed consent?
True informed consent is a process of managing a patient's expectations; it is not just a signature on a document. The physician must then provide sufficient information to the patient so that a reasonable and informed decision regarding a treatment plan can be made. This physician responsibility cannot be delegated.
What are informed consent laws?
Informed Consent Law covers the legal aspect regarding an individual's right to be informed of and consent to a procedure or treatment suggested by a physician or professional. The patient can then make an informed decision to accept or refuse the treatment or procedure.
Is informed consent necessary?
Informed consent serves as a valuable tool in asserting proper regulations in clinical trials, as well as providing assurance of safety for the patient. In situations such as emergency research or research with minimal risk to the subject, informed consent is not absolutely necessary.
Can nurse practitioners obtain informed consent?
Informed consent is a concept with historic, legal, and ethical implications, but sometimes ill-defined parameters for clinical application. Nurse practitioners, as patient advocates and skilled communicators, are well positioned to participate in the informed consent process.
Who can witness an informed consent?
Who Should Serve as a Witness to the Informed Consent Process? Ideally, the witness should be someone who has accompanied the patient to the practice, and has been present when the provider discusses the recommended treatment or procedure with the patient.
What type of role should nurses have securing informed consent?
Informed consent, that is shared decision-making, is an important communication process between patients and professional caregivers. We consider the roles of nursing for informed consent are as follows: 1. Support of patients' decision-making; 1) promotion of patients' understanding of their own situation, a.
What is informed consent in surgery?
Informed consent for surgery entails what surgeons communicate to their patients about the proposed surgery and is a key element in the trust patients have in surgeons. It is of increasing importance, and we must keep up to date with patient and legal expectations.
How do you write a consent form?
Writing a Consent Form A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: Use University of Wollongong/AHS letterhead. 1. Provide the title of the research project, the researcher(s) name, supervisor's name (for 2.
What is a participant consent form?
A consent form is not simply about a person giving you permission to involve them in research, it is an agreement between the researcher and the research participant outlining the roles and responsibilities they are taking towards one another throughout the whole of the research process.
How do you write a consent questionnaire?
Information about the researcher or research institution.
Research purpose.
Potential risks & hazards.
Duration subject's participation.
Contact information.
A statement that the agreement is voluntary.
Do you need a consent form for surveys?
The informed consent process is a basic ethical obligation for researchers. The consent document is the only record linking the subject with the research. Most survey research meets the requirements for waivers of signed consent, because surveys conducted outside a research context rarely require written consent.
How do I write an authorization letter?
On the top left-hand corner of the page put down the name, address, and date.
Below the name of the letter writer, it is important to include the name of the recipient and the relevant address.
The letter then will open with Dear, Mr/Ms.
How do I write a simple authorization letter?
Identify yourself by yourself as accurately as possible.
Introduce the person or entity to whom you wish to grant authority.
Mention their name, their ID number, and how they relate to you.
Specify the scope of the authority, that is the allowed actions.
eSignature workflows made easy
Sign, send for signature, and track documents in real-time with signNow.