Digital Signature Detailed Medical Consent For Free




Users trust to manage documents on pdfFiller platform
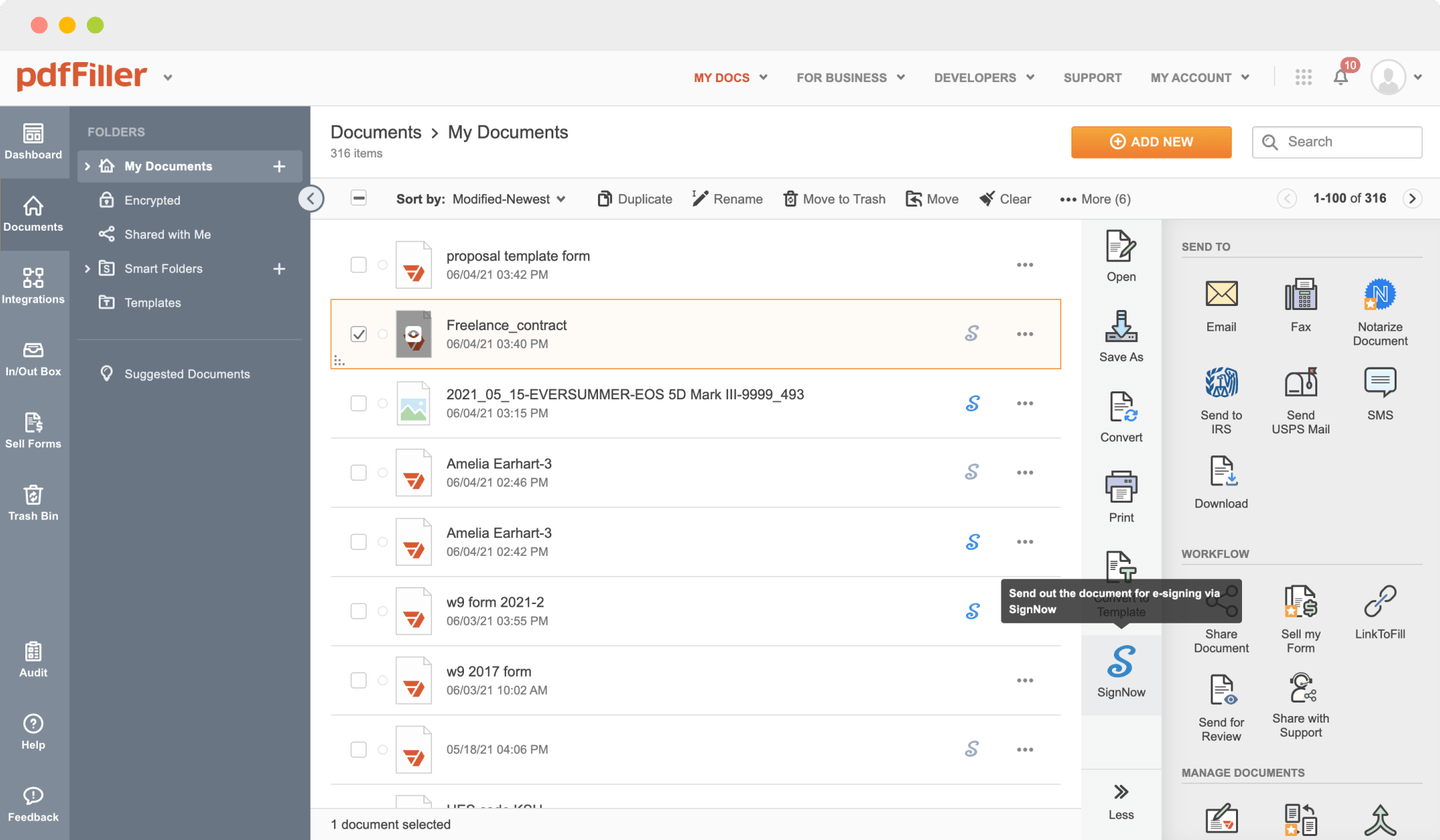
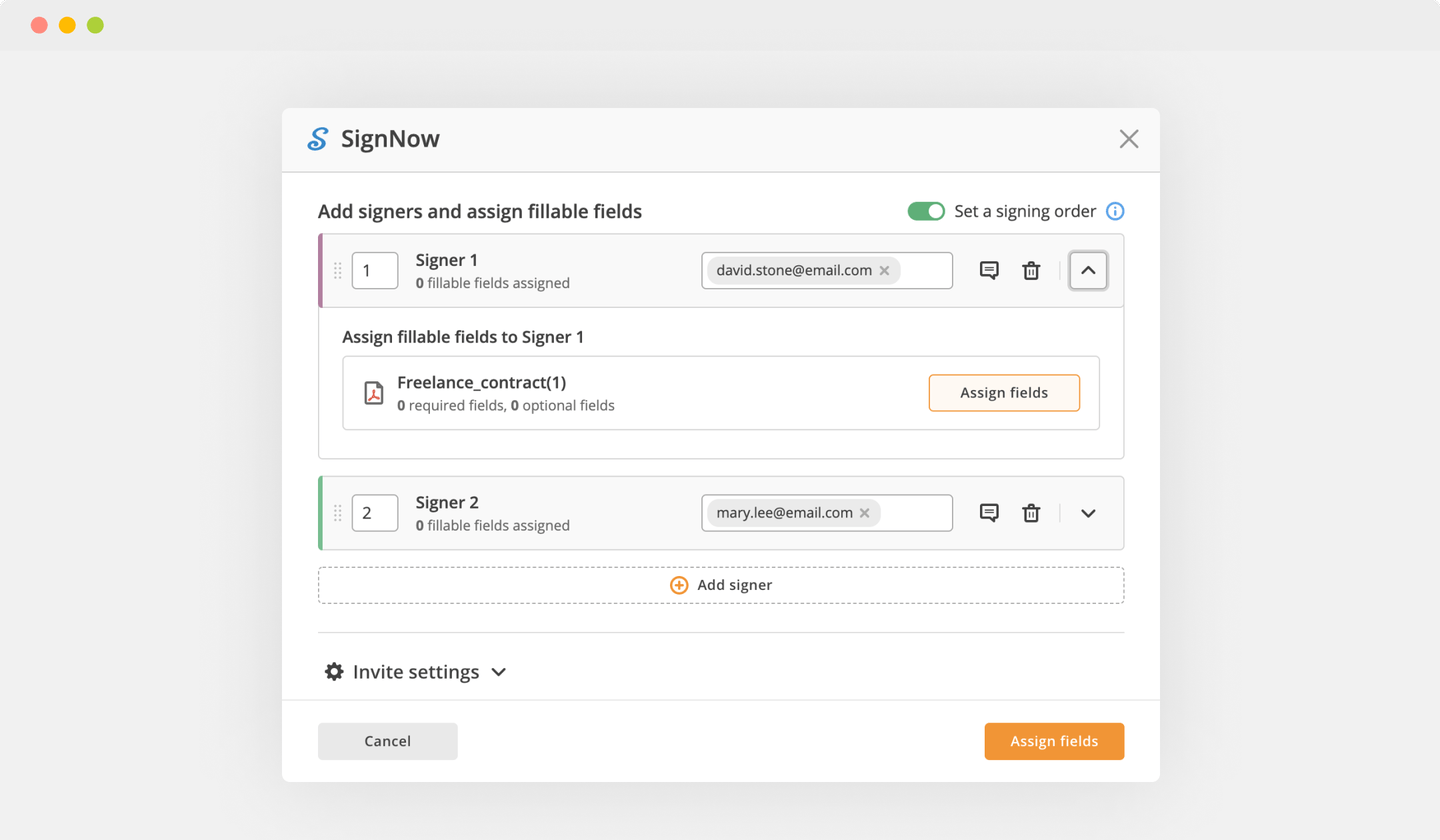
Send documents for eSignature with signNow
Watch a short video walkthrough on how to add an Digital Signature Detailed Medical Consent
pdfFiller scores top ratings in multiple categories on G2
Create a legally-binding Digital Signature Detailed Medical Consent in minutes
pdfFiller enables you to handle Digital Signature Detailed Medical Consent like a pro. Regardless of the system or device you run our solution on, you'll enjoy an instinctive and stress-free way of executing documents.
The entire pexecution flow is carefully safeguarded: from uploading a document to storing it.
Here's the best way to create Digital Signature Detailed Medical Consent with pdfFiller:
Choose any available way to add a PDF file for signing.
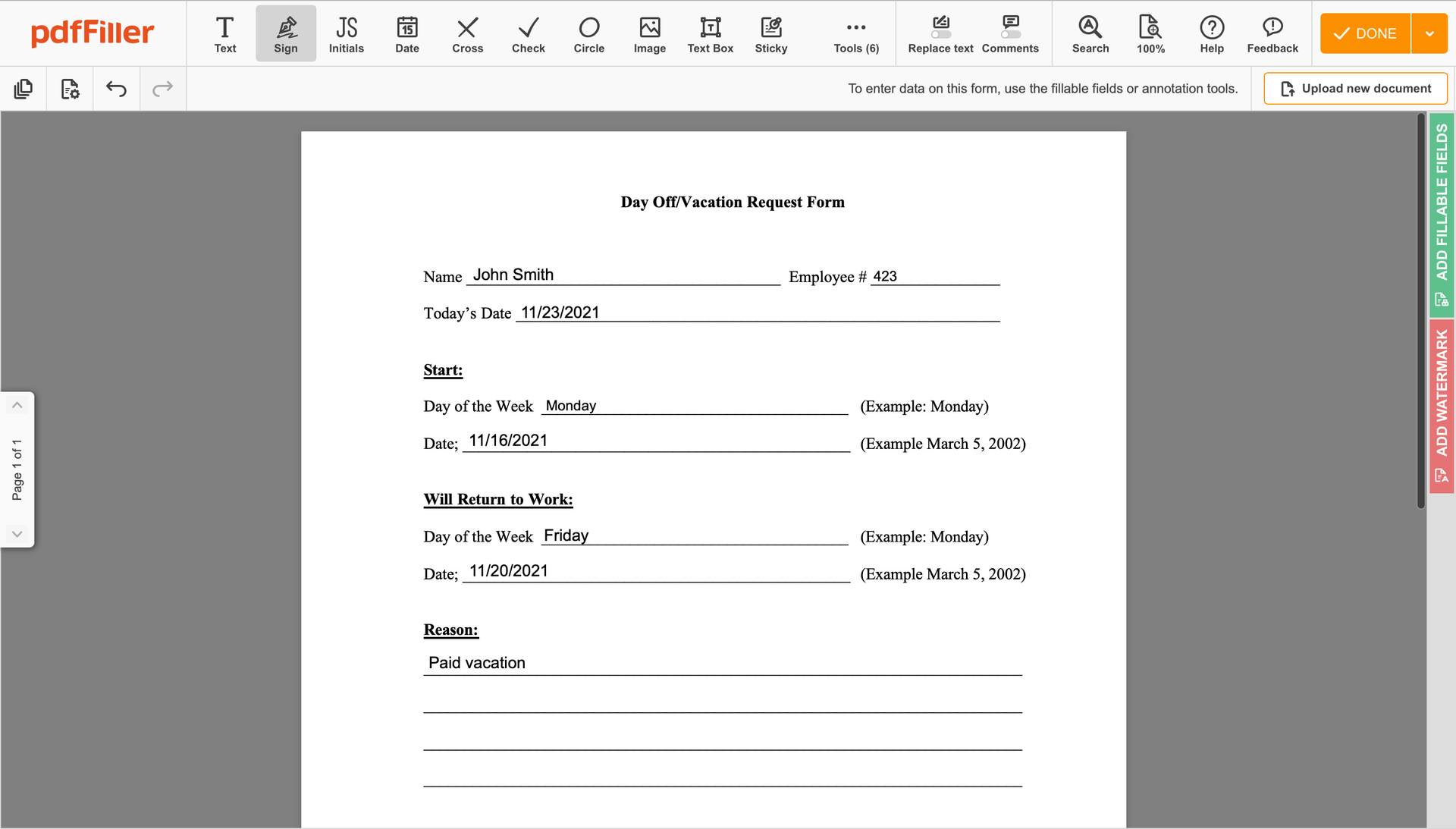
Use the toolbar at the top of the interface and choose the Sign option.
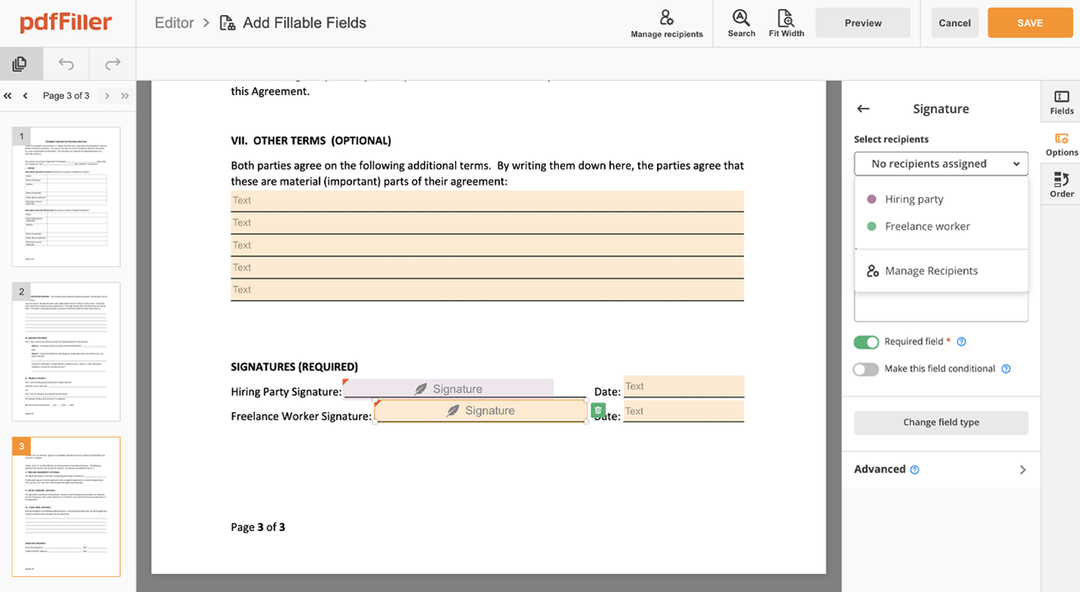
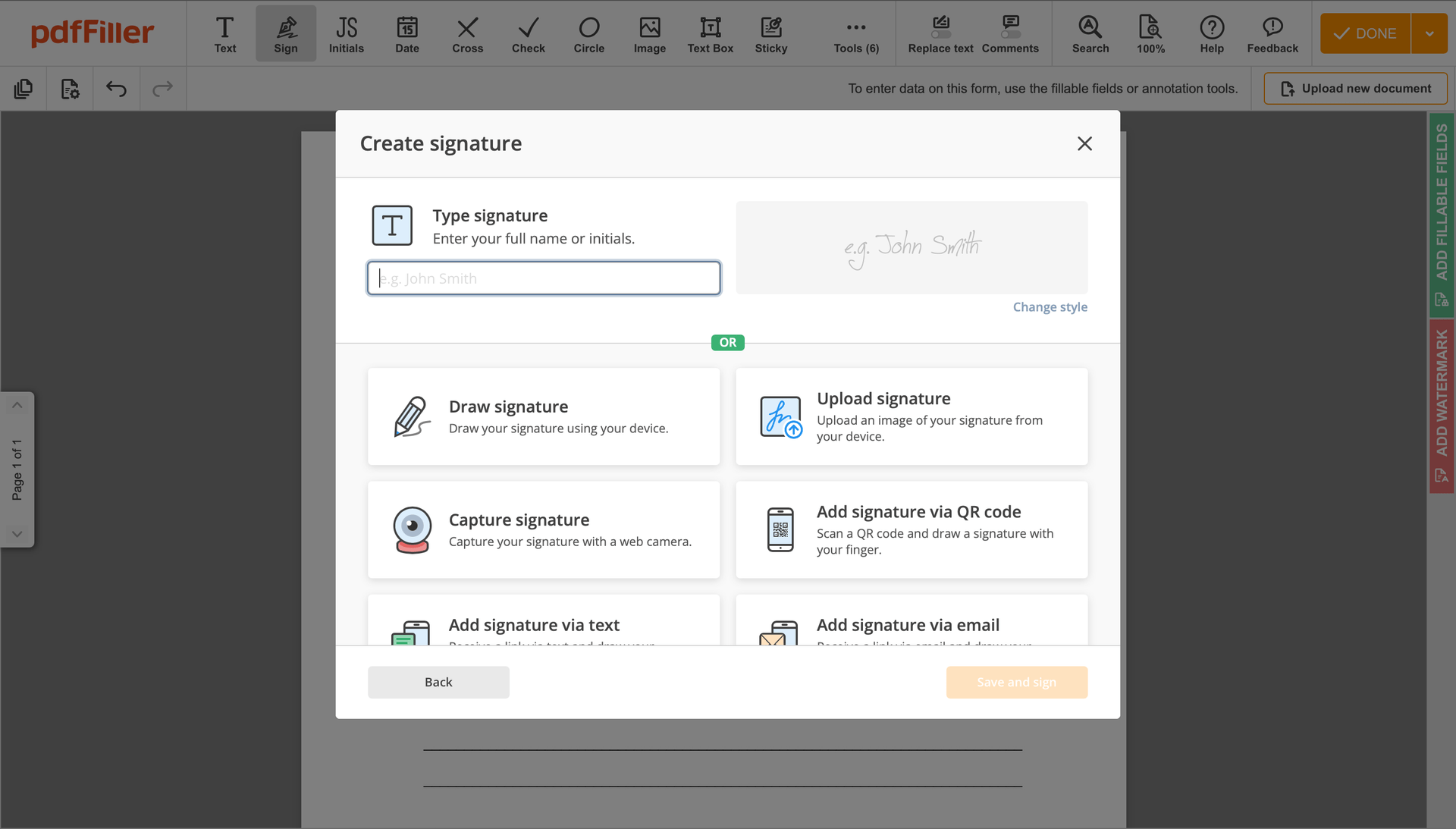
You can mouse-draw your signature, type it or upload an image of it - our solution will digitize it automatically. As soon as your signature is set up, click Save and sign.
Click on the form area where you want to add an Digital Signature Detailed Medical Consent. You can drag the newly created signature anywhere on the page you want or change its configurations. Click OK to save the adjustments.
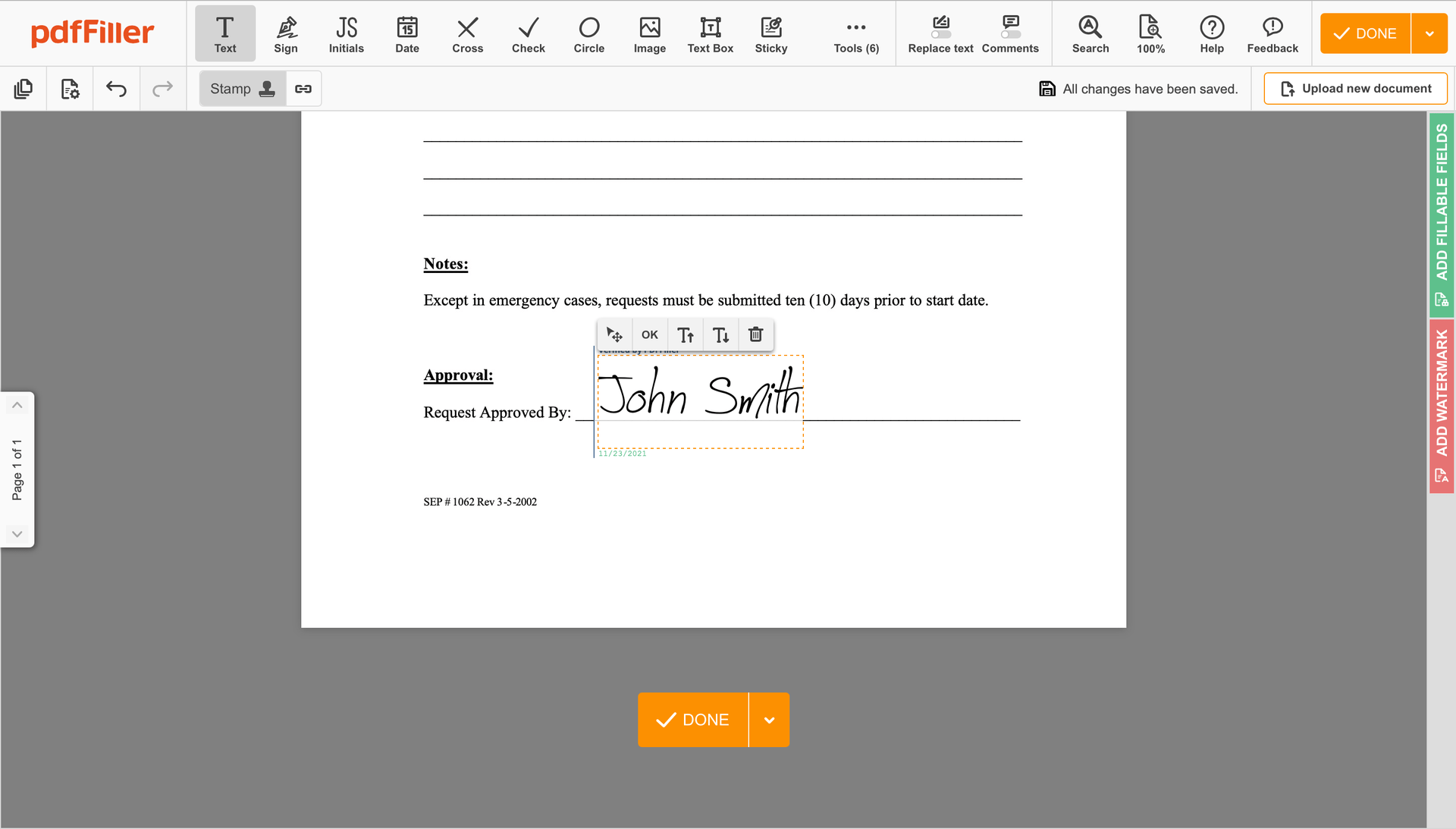
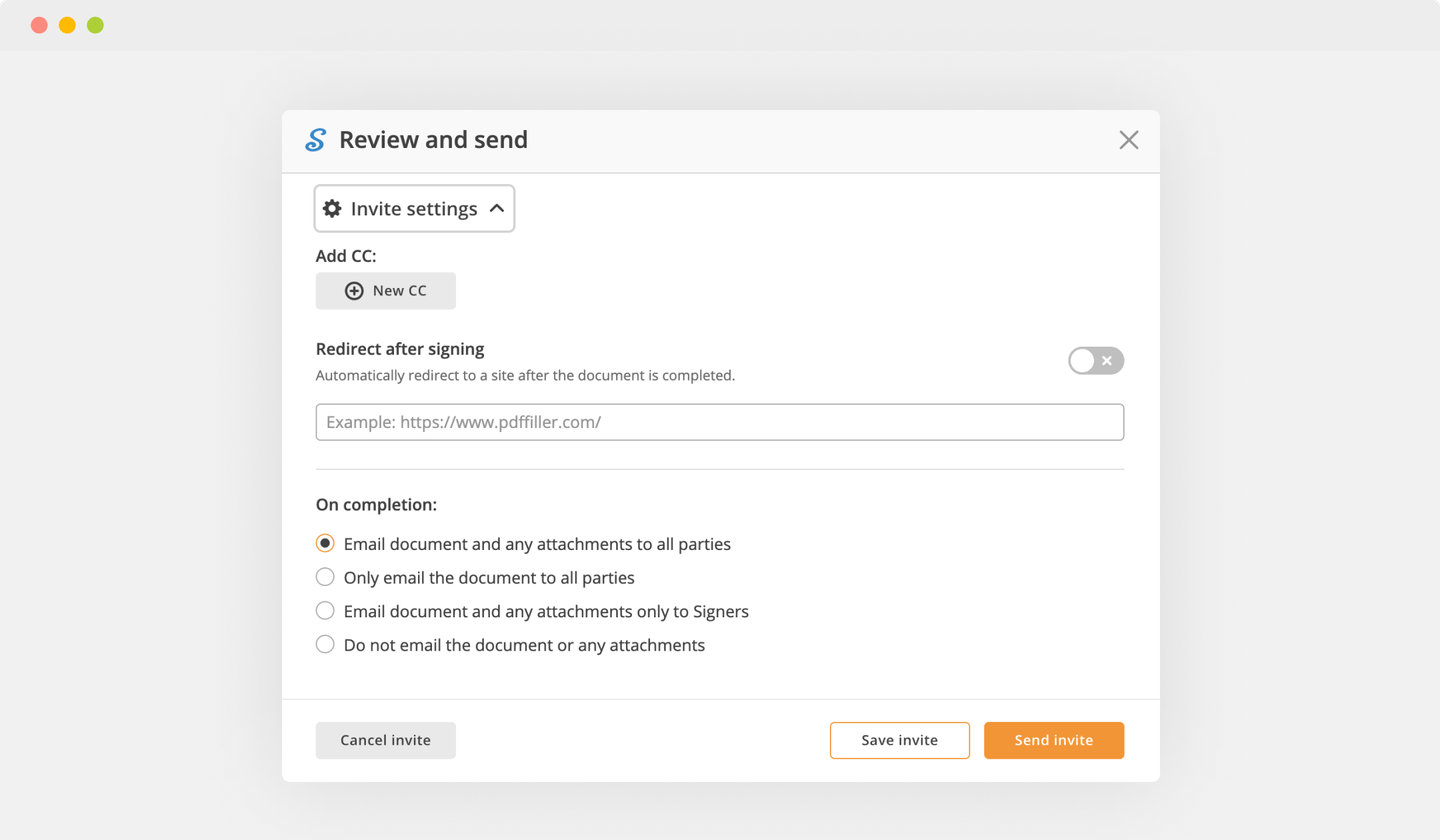
As soon as your document is all set, hit the DONE button in the top right area.
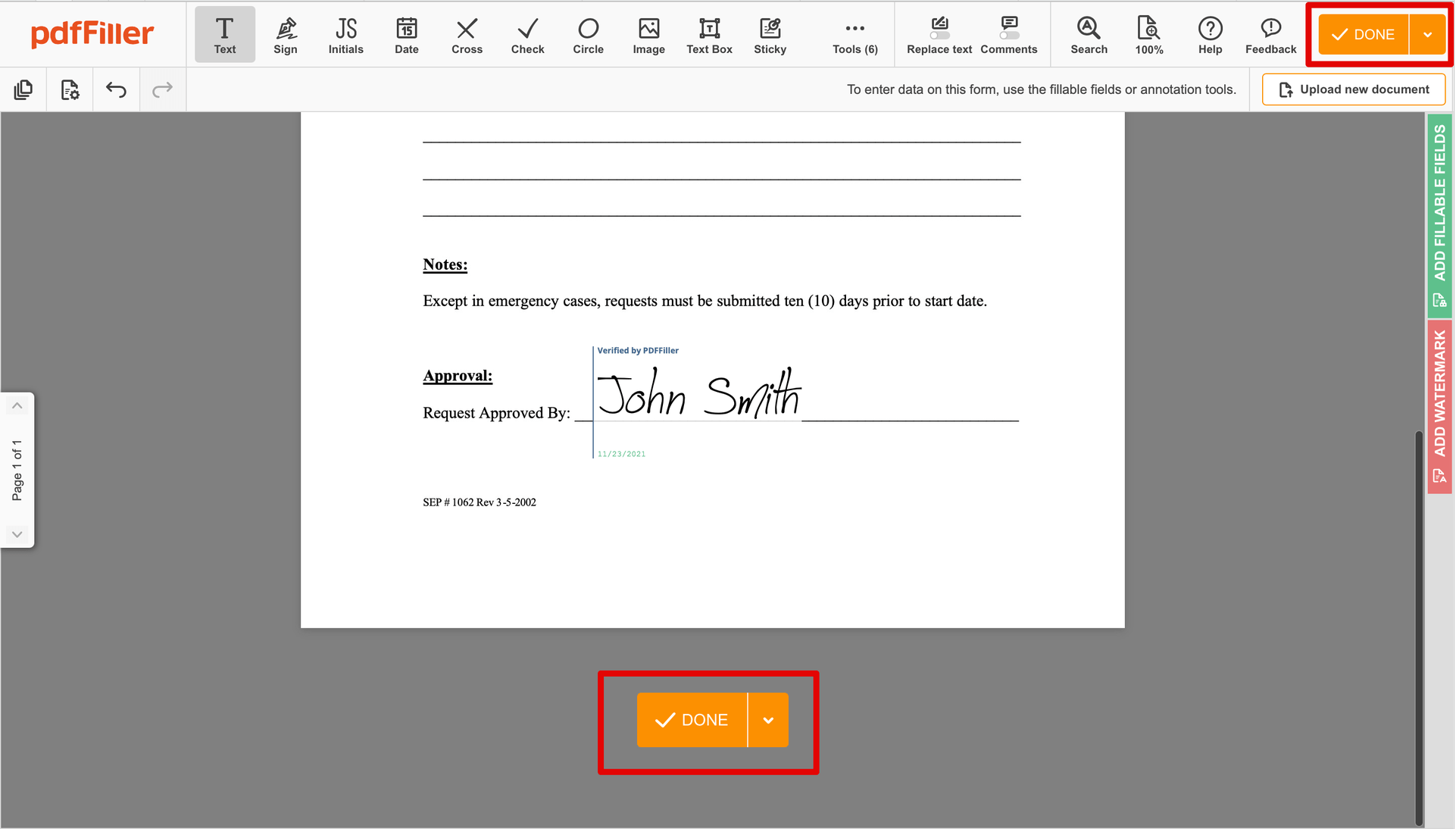
Once you're through with signing, you will be redirected to the Dashboard.
Use the Dashboard settings to download the completed copy, send it for further review, or print it out.
Stuck working with different programs for managing documents? Try our all-in-one solution instead. Use our tool to make the process fast and simple. Create forms, contracts, make document template sand other useful features, without leaving your browser. You can use Digital Signature Detailed Medical Consent right away, all features, like signing orders, alerts, attachment and payment requests, are available instantly. Have an advantage over other applications.
How to edit a PDF document using the pdfFiller editor:
How to Send a PDF for eSignature
What our customers say about pdfFiller