Signatory Detailed Medical Consent For Free




Join the world’s largest companies
How to Send a PDF for eSignature









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Signatory Detailed Medical Consent Feature
The Signatory Detailed Medical Consent feature streamlines the process of obtaining informed consent from patients. This tool helps healthcare providers manage consent efficiently, ensuring that all necessary information is documented clearly and accurately.
Key Features
Potential Use Cases and Benefits
With the Signatory Detailed Medical Consent feature, you can solve the challenge of navigating complex consent processes. This tool reduces the administrative burden on your team, while ensuring that patients are informed and engaged in their care. Ultimately, you promote a culture of transparency and trust, leading to improved patient satisfaction and outcomes.
Add a legally-binding Signatory Detailed Medical Consent with no hassle
pdfFiller allows you to deal with Signatory Detailed Medical Consent like a pro. No matter the system or device you use our solution on, you'll enjoy an easy-to-use and stress-free method of executing paperwork.
The entire signing process is carefully safeguarded: from adding a file to storing it.
Here's the best way to create Signatory Detailed Medical Consent with pdfFiller:
Select any available option to add a PDF file for signing.
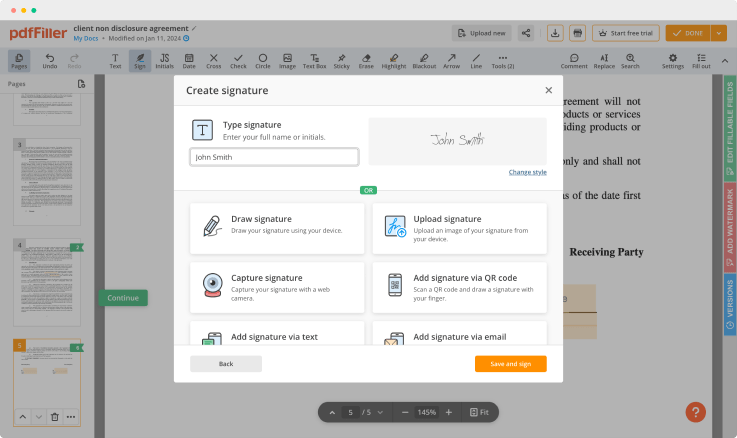
Use the toolbar at the top of the page and select the Sign option.
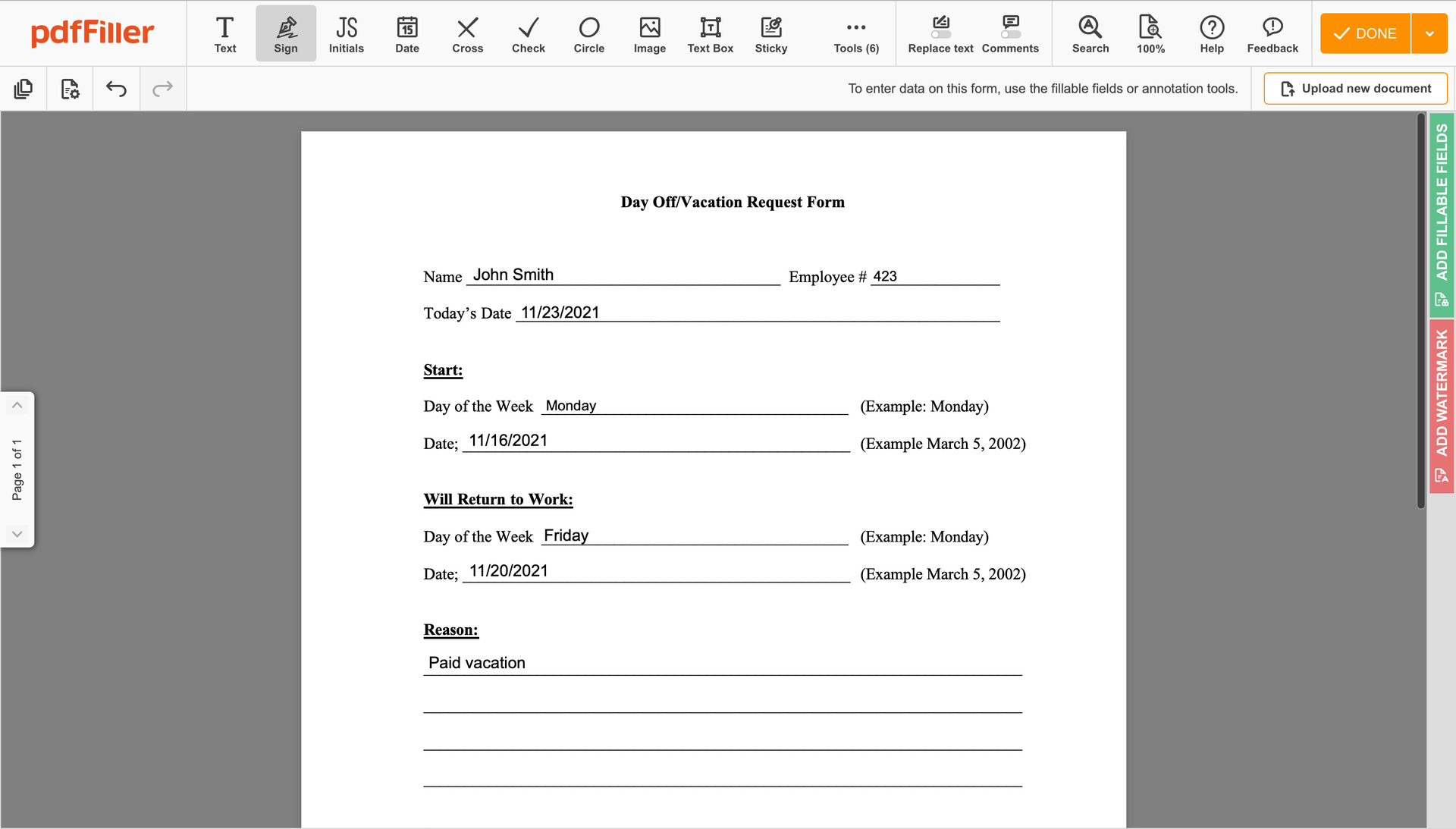
You can mouse-draw your signature, type it or upload a photo of it - our solution will digitize it in a blink of an eye. As soon as your signature is set up, click Save and sign.
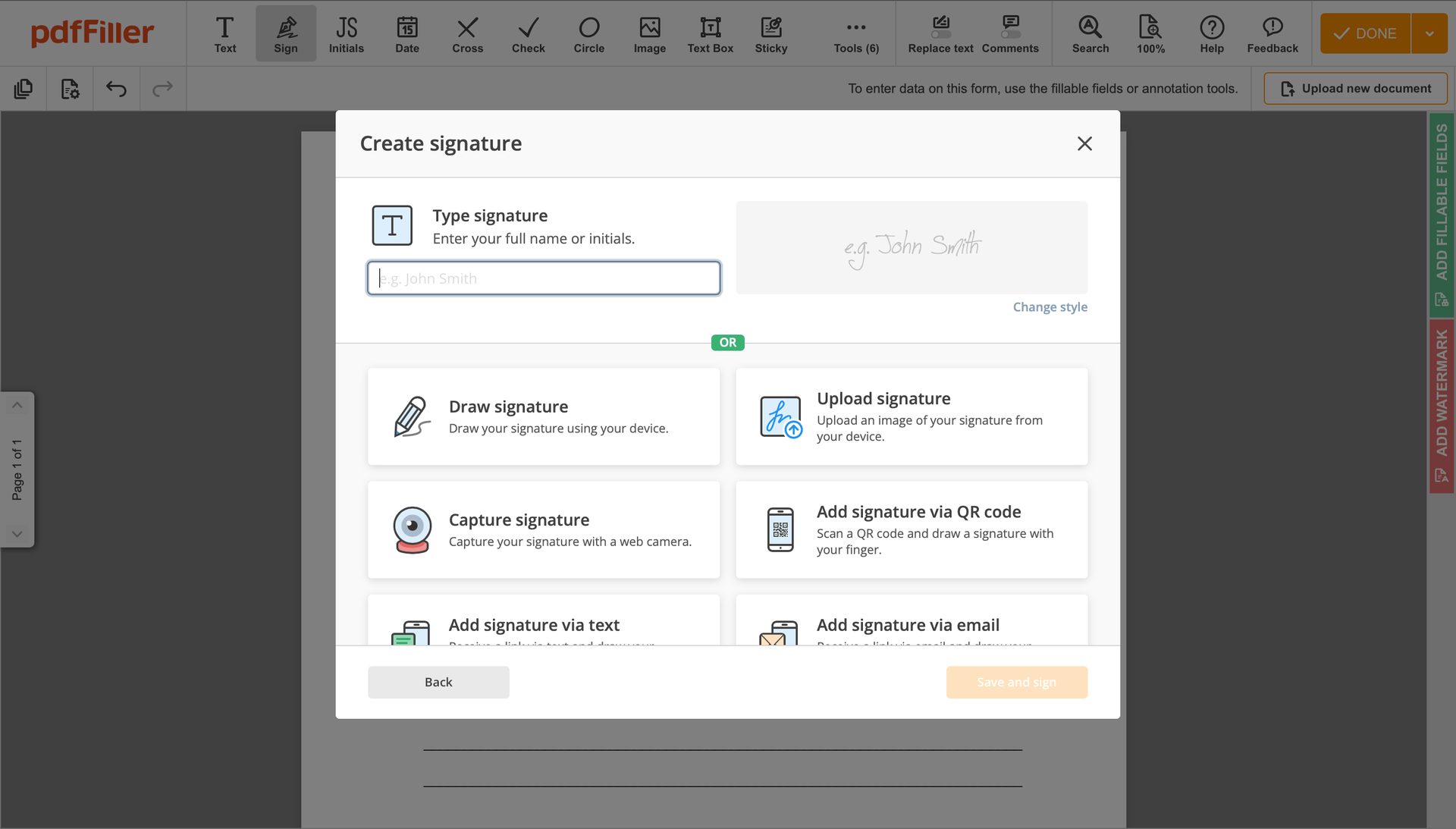
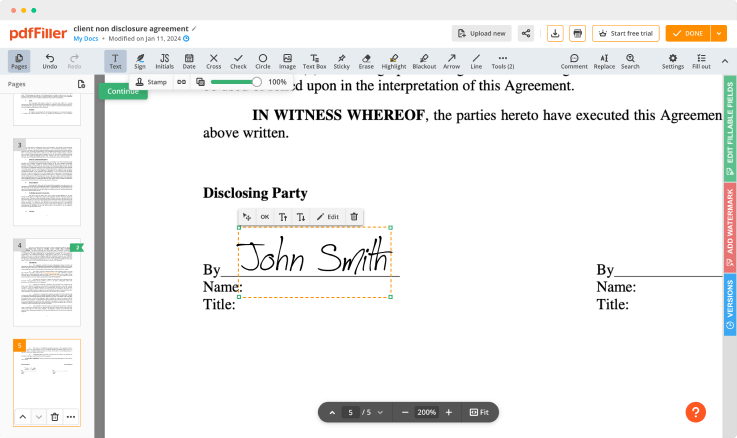
Click on the form place where you want to put an Signatory Detailed Medical Consent. You can drag the newly generated signature anywhere on the page you want or change its settings. Click OK to save the changes.
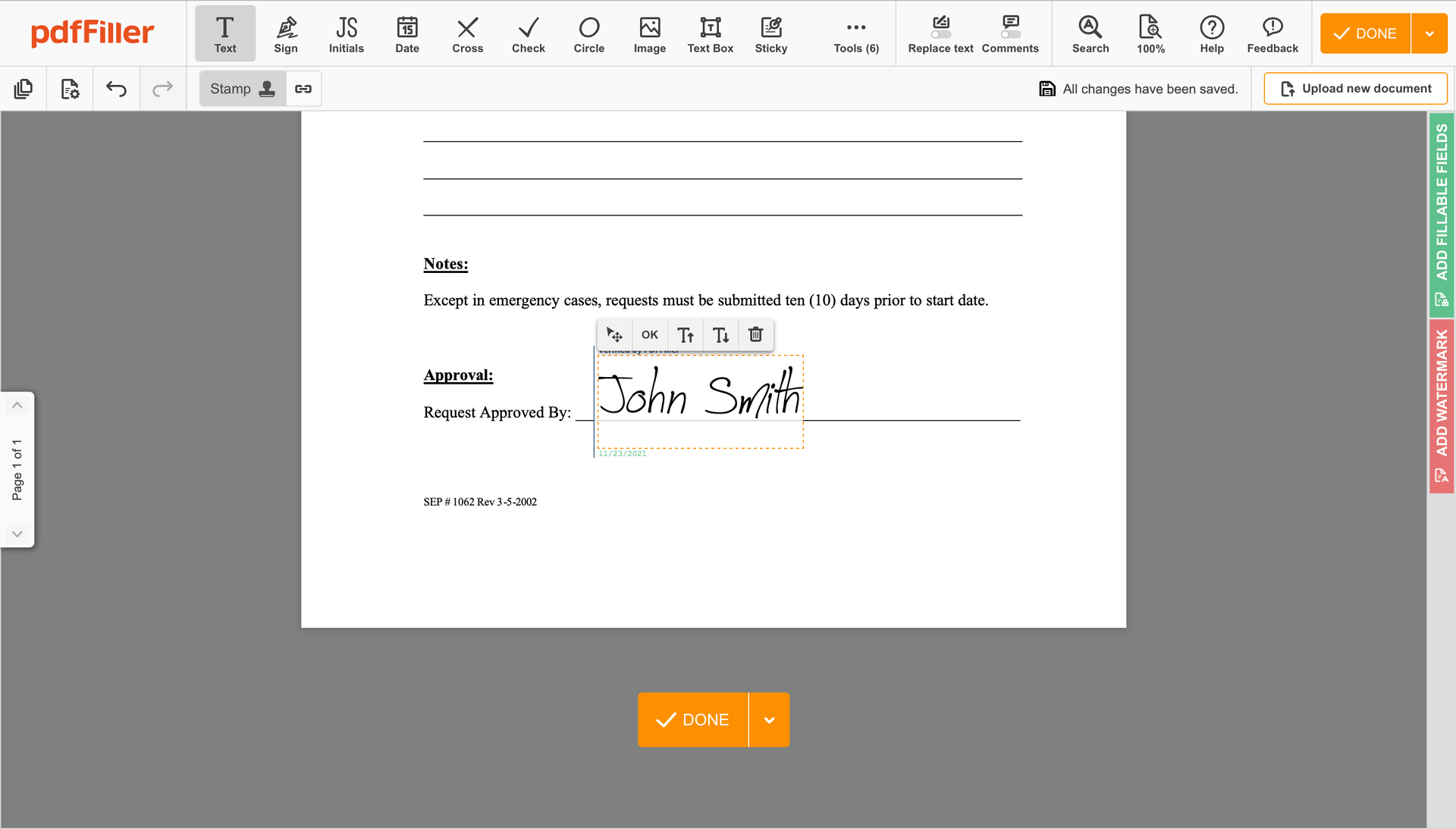
Once your form is all set, click on the DONE button in the top right corner.
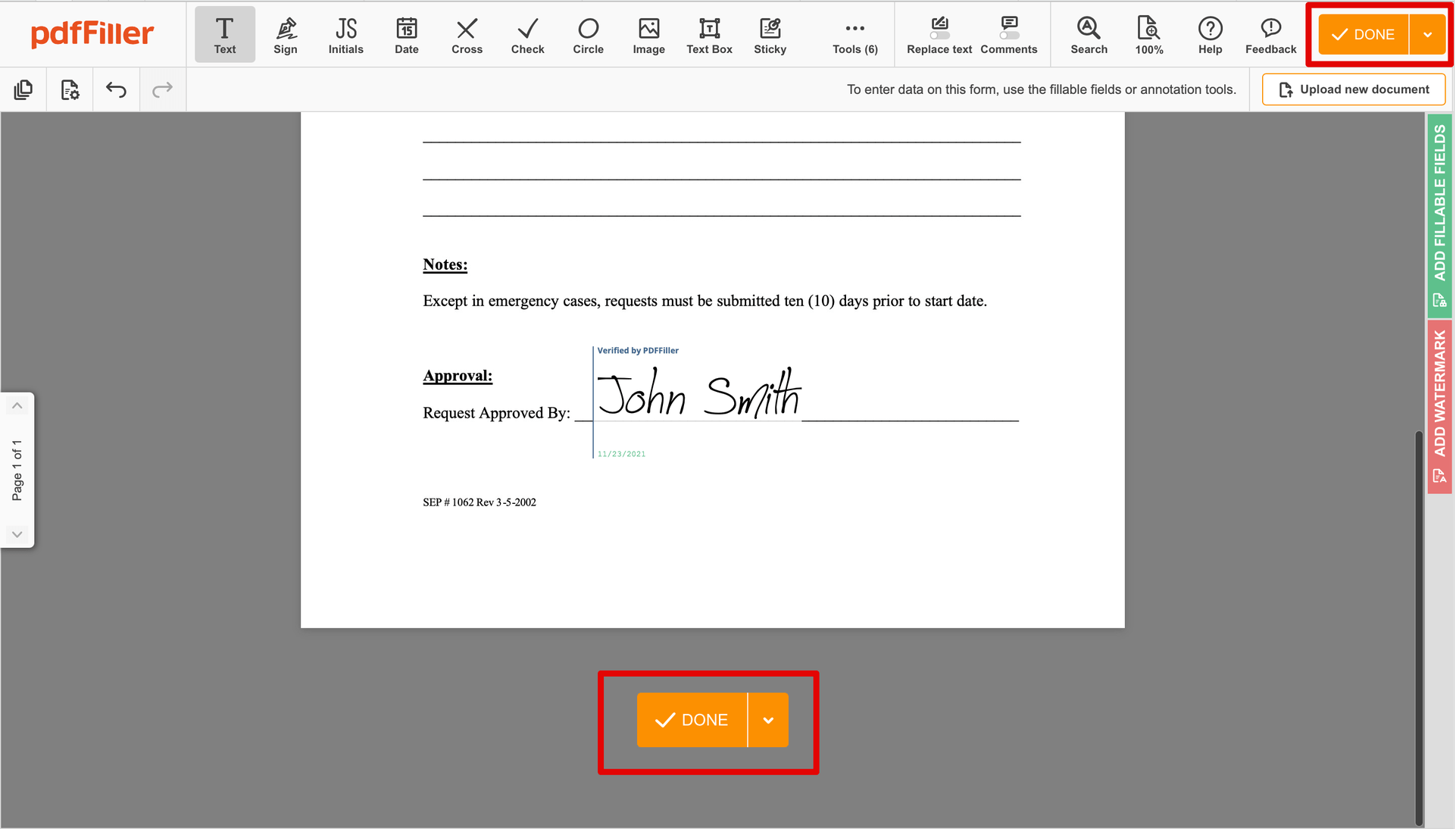
Once you're done with certifying your paperwork, you will be redirected to the Dashboard.
Utilize the Dashboard settings to download the executed copy, send it for further review, or print it out.
Still using multiple applications to sign and manage your documents? We've got a solution for you. Use our document management tool for the fast and efficient workflow. Create document templates completely from scratch, modify existing form sand even more features, within your browser. Plus, you can use Signatory Detailed Medical Consent and add high-quality features like orders signing, reminders, requests, easier than ever. Pay as for a basic app, get the features as of pro document management tools.
How to edit a PDF document using the pdfFiller editor:
For pdfFiller’s FAQs
Ready to try pdfFiller's? Signatory Detailed Medical Consent