Sign Consent For Free




Users trust to manage documents on pdfFiller platform
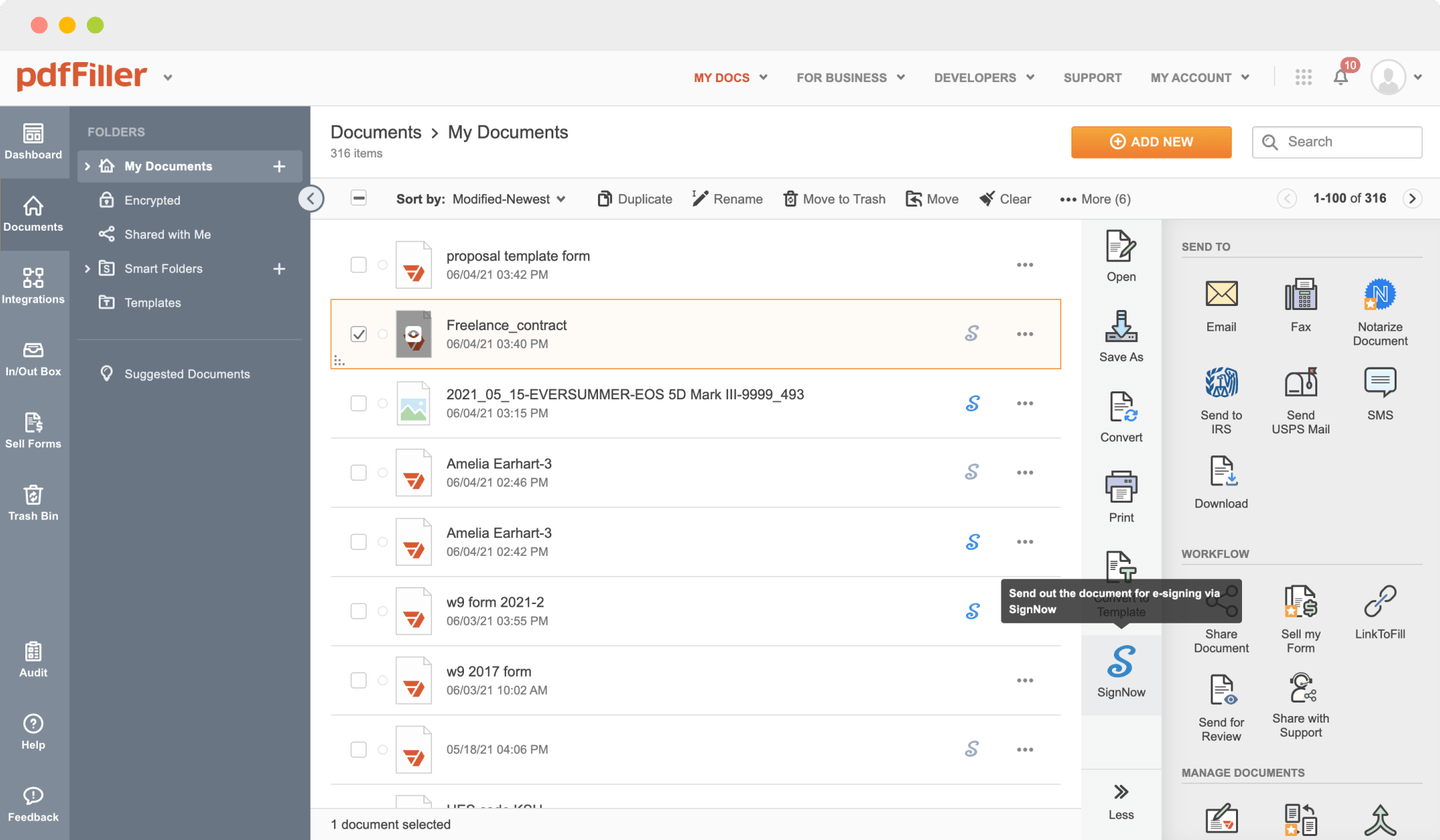
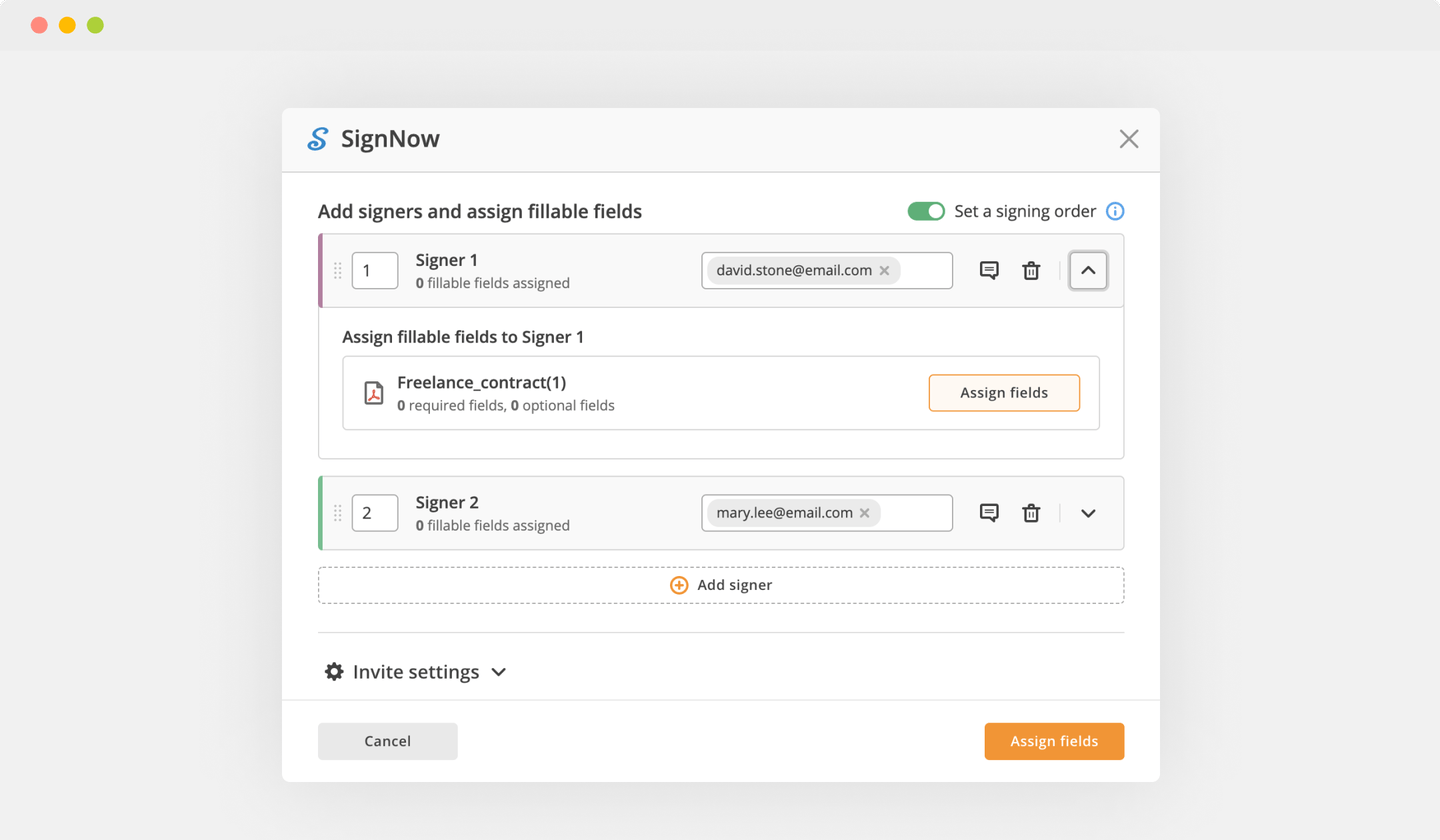
Send documents for eSignature with signNow
Watch a quick video tutorial on how to Sign Consent
pdfFiller scores top ratings in multiple categories on G2
Sign Consent in minutes
pdfFiller enables you to Sign Consent in no time. The editor's handy drag and drop interface ensures quick and user-friendly signing on any operaring system.
Signing PDFs online is a quick and secure way to verify documents anytime and anywhere, even while on the fly.
See the step-by-step instructions on how to Sign Consent electronically with pdfFiller:
Upload the form for eSignature to pdfFiller from your device or cloud storage.
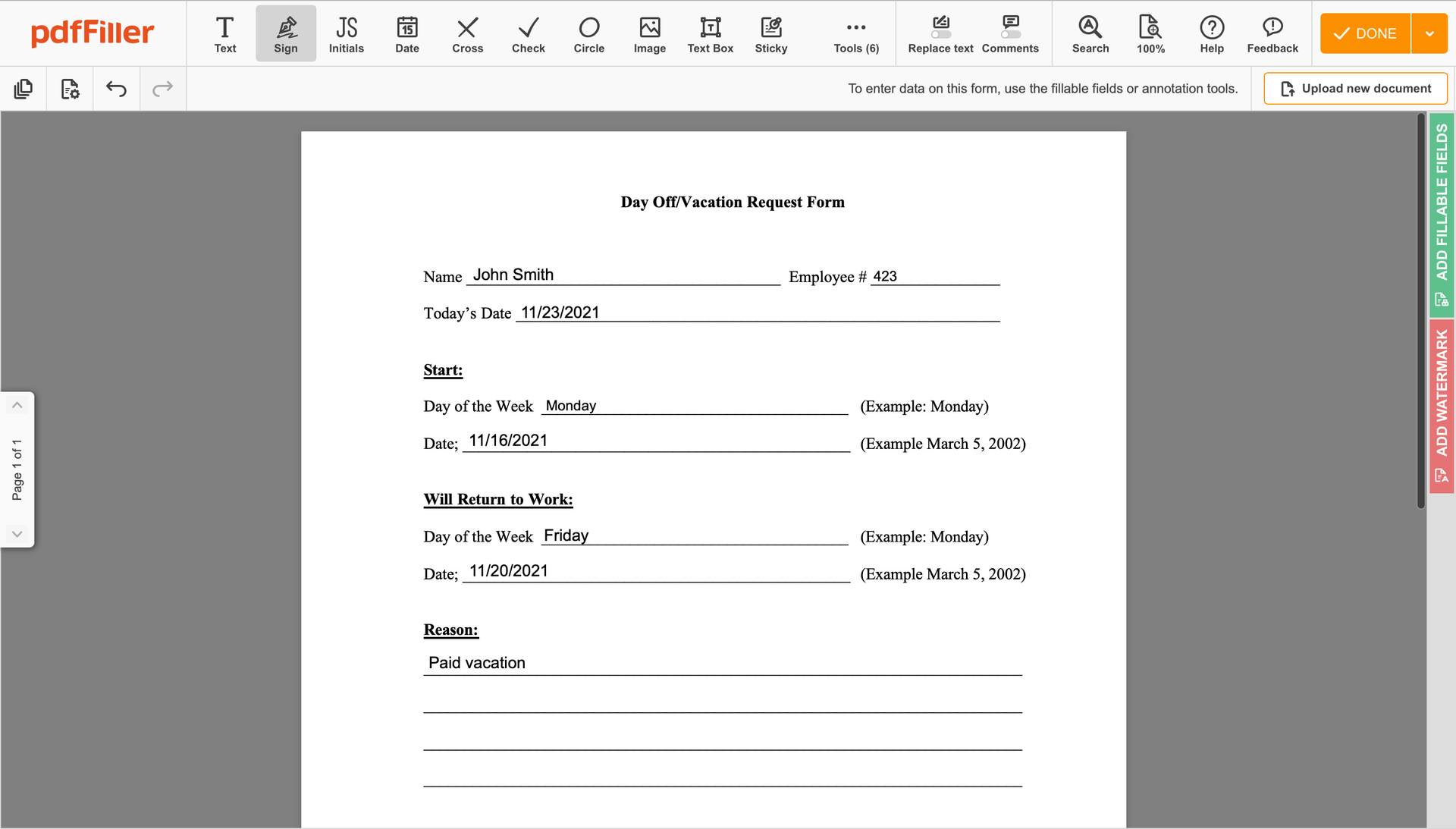
Once the document opens in the editor, click Sign in the top toolbar.
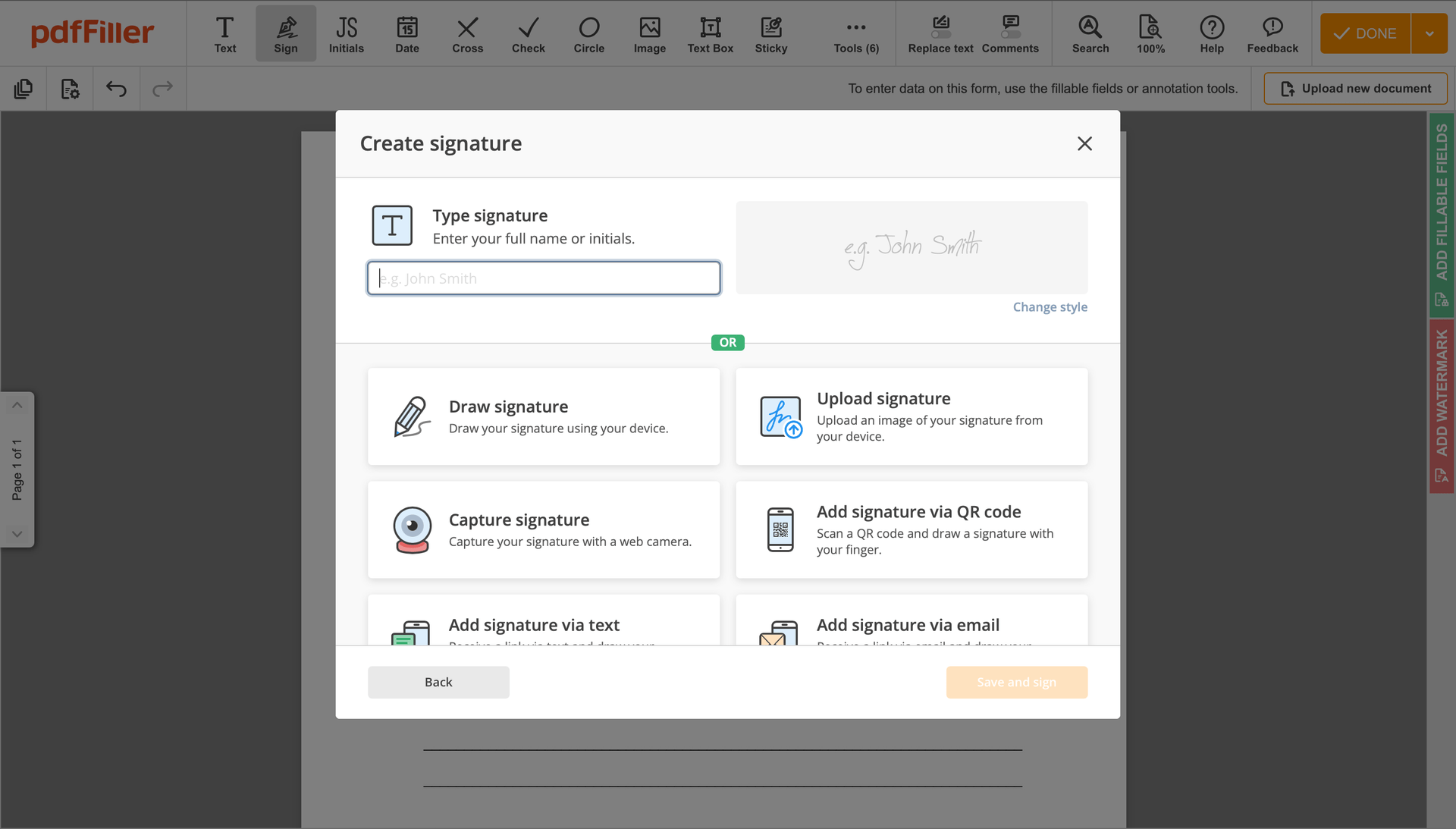
Create your electronic signature by typing, drawing, or uploading your handwritten signature's image from your device. Then, hit Save and sign.
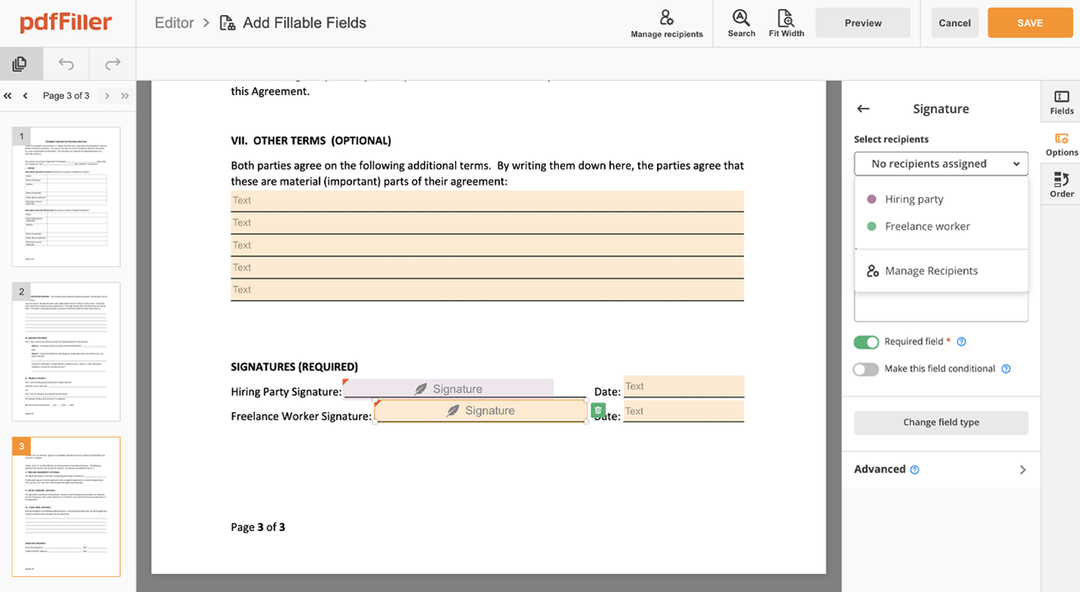
Click anywhere on a document to Sign Consent. You can move it around or resize it using the controls in the floating panel. To use your signature, hit OK.
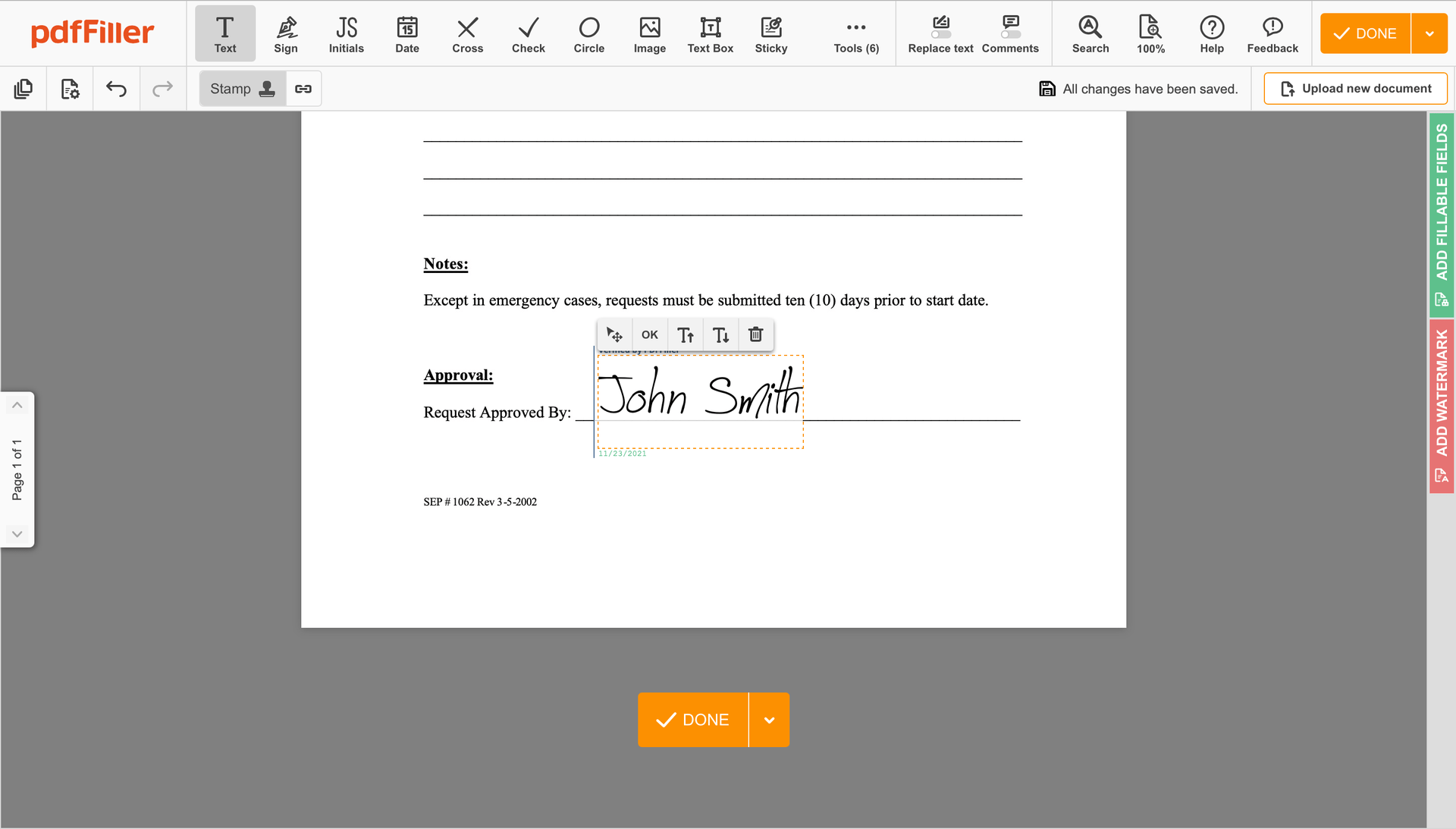
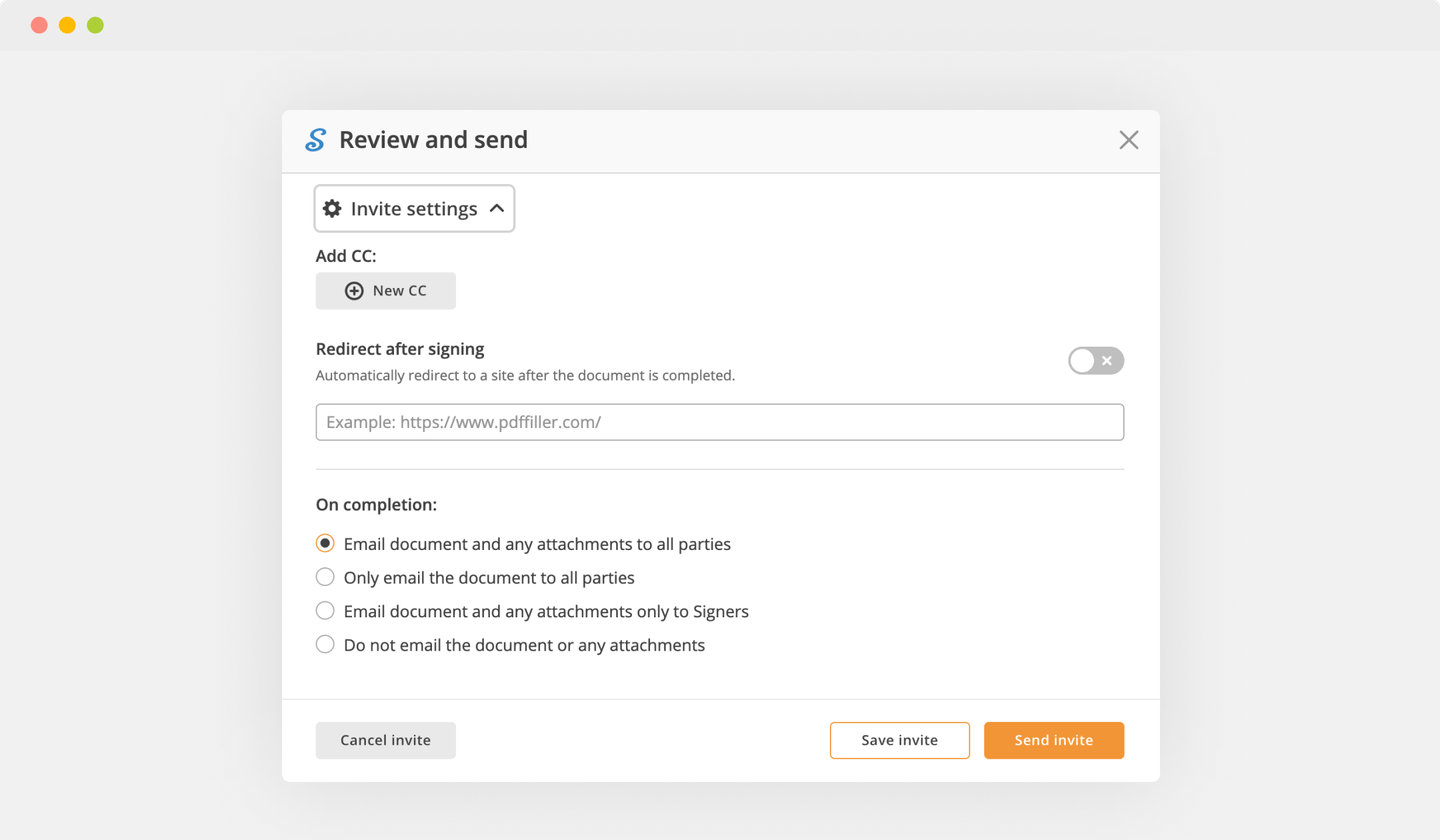
Finish up the signing session by clicking DONE below your form or in the top right corner.
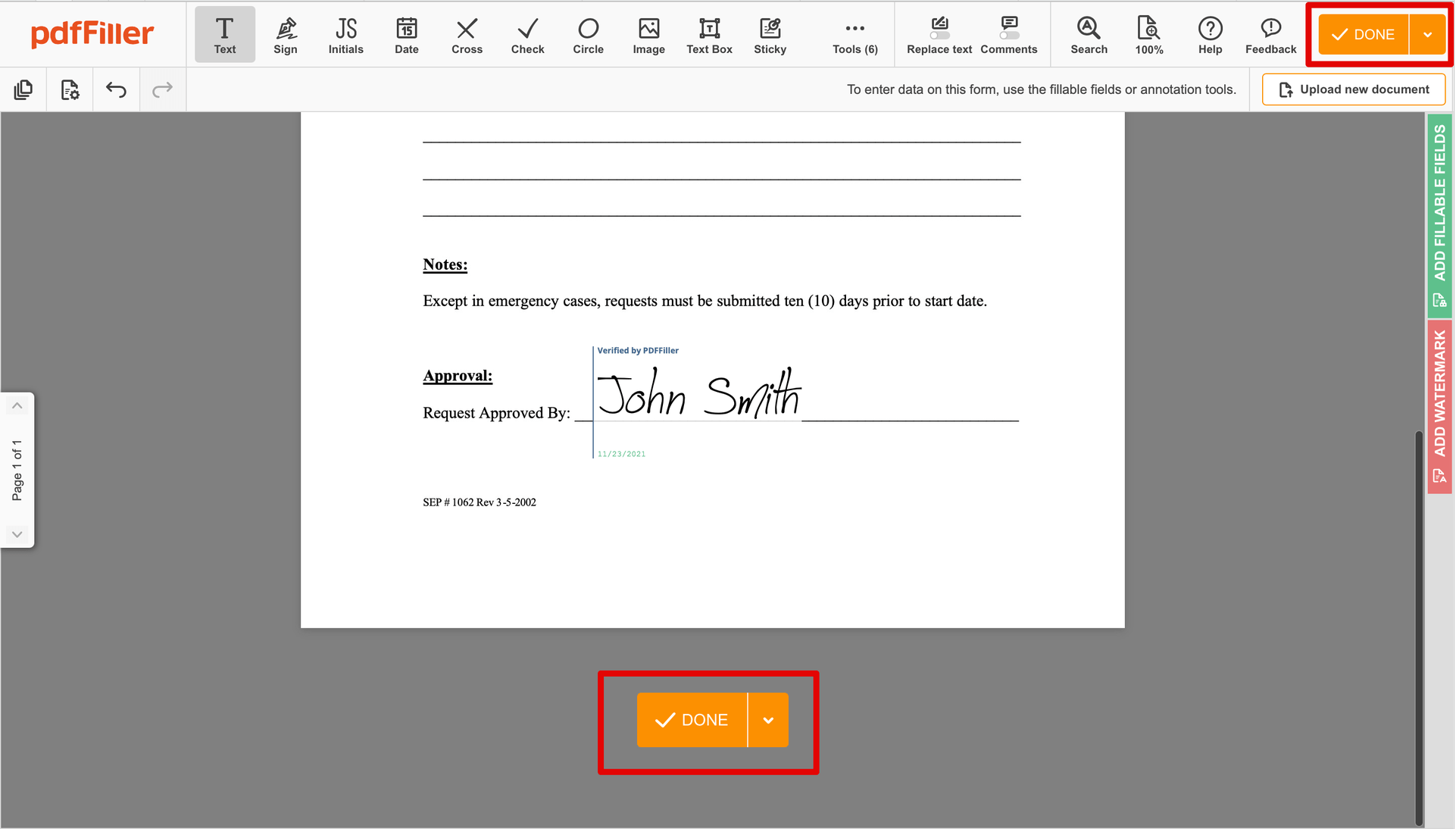
After that, you'll go back to the pdfFiller dashboard. From there, you can get a completed copy, print the document, or send it to other people for review or approval.
Stuck working with multiple applications for creating and managing documents? We have a solution for you. Use our editor to make the process fast and efficient. Create document templates from scratch, edit existing form sand other features, without leaving your browser. You can use Sign Consent right away, all features, like signing orders, alerts, attachment and payment requests, are available instantly. Pay as for a basic app, get the features as of pro document management tools. The key is flexibility, usability and customer satisfaction.
How to edit a PDF document using the pdfFiller editor:
How to Send a PDF for eSignature
What our customers say about pdfFiller