Redline Clinical Trial Agreement Template




Users trust to manage documents on pdfFiller platform
Watch a quick video tutorial on how to Redline Clinical Trial Agreement Template
pdfFiller scores top ratings in multiple categories on G2
Redline Clinical Trial Agreement Template in minutes
pdfFiller enables you to Redline Clinical Trial Agreement Template quickly. The editor's hassle-free drag and drop interface ensures quick and user-friendly signing on any device.
Signing PDFs online is a quick and safe way to validate documents at any time and anywhere, even while on the go.
Go through the step-by-step instructions on how to Redline Clinical Trial Agreement Template electronically with pdfFiller:
Add the document you need to sign to pdfFiller from your device or cloud storage.
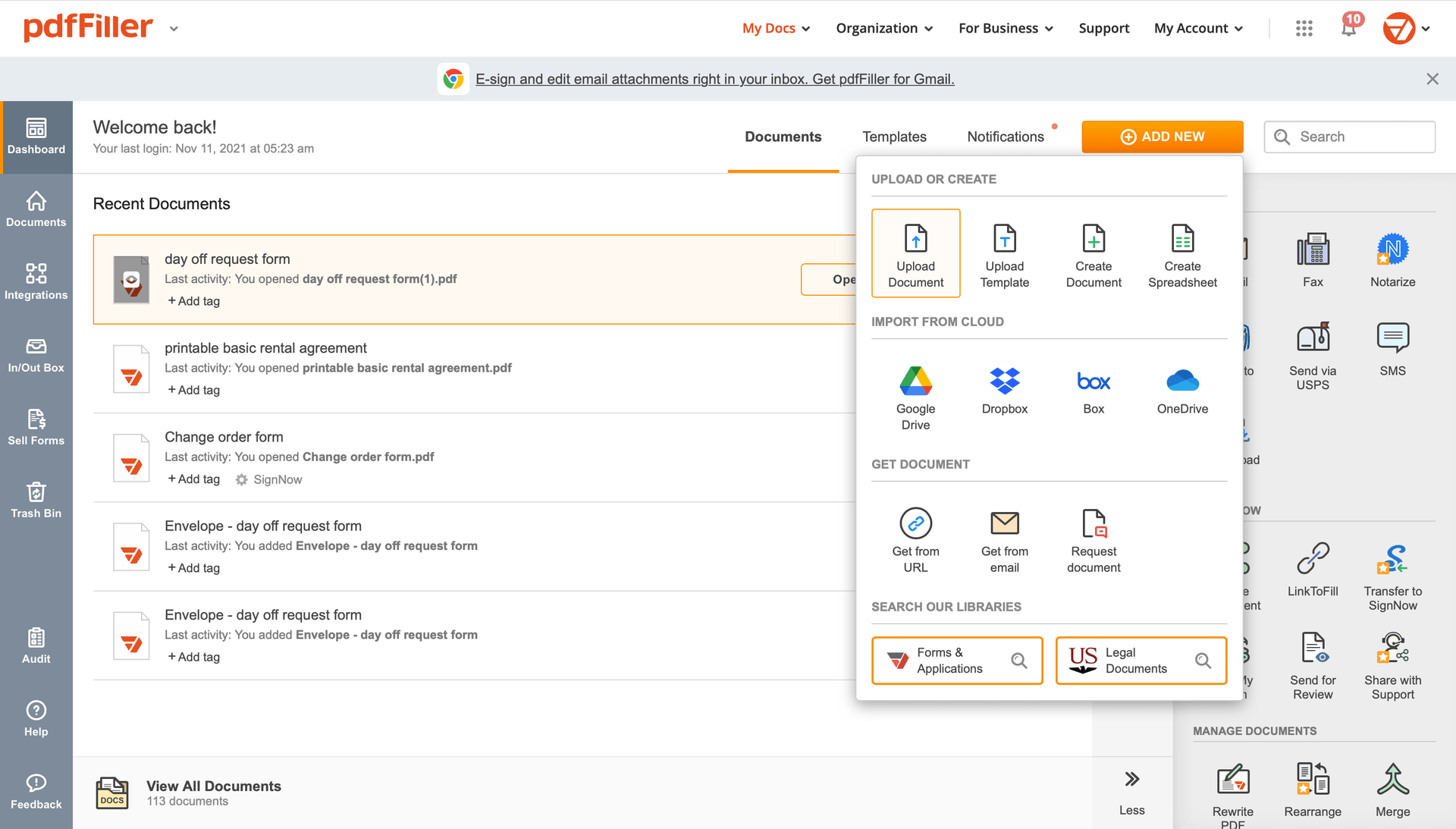
Once the file opens in the editor, hit Sign in the top toolbar.
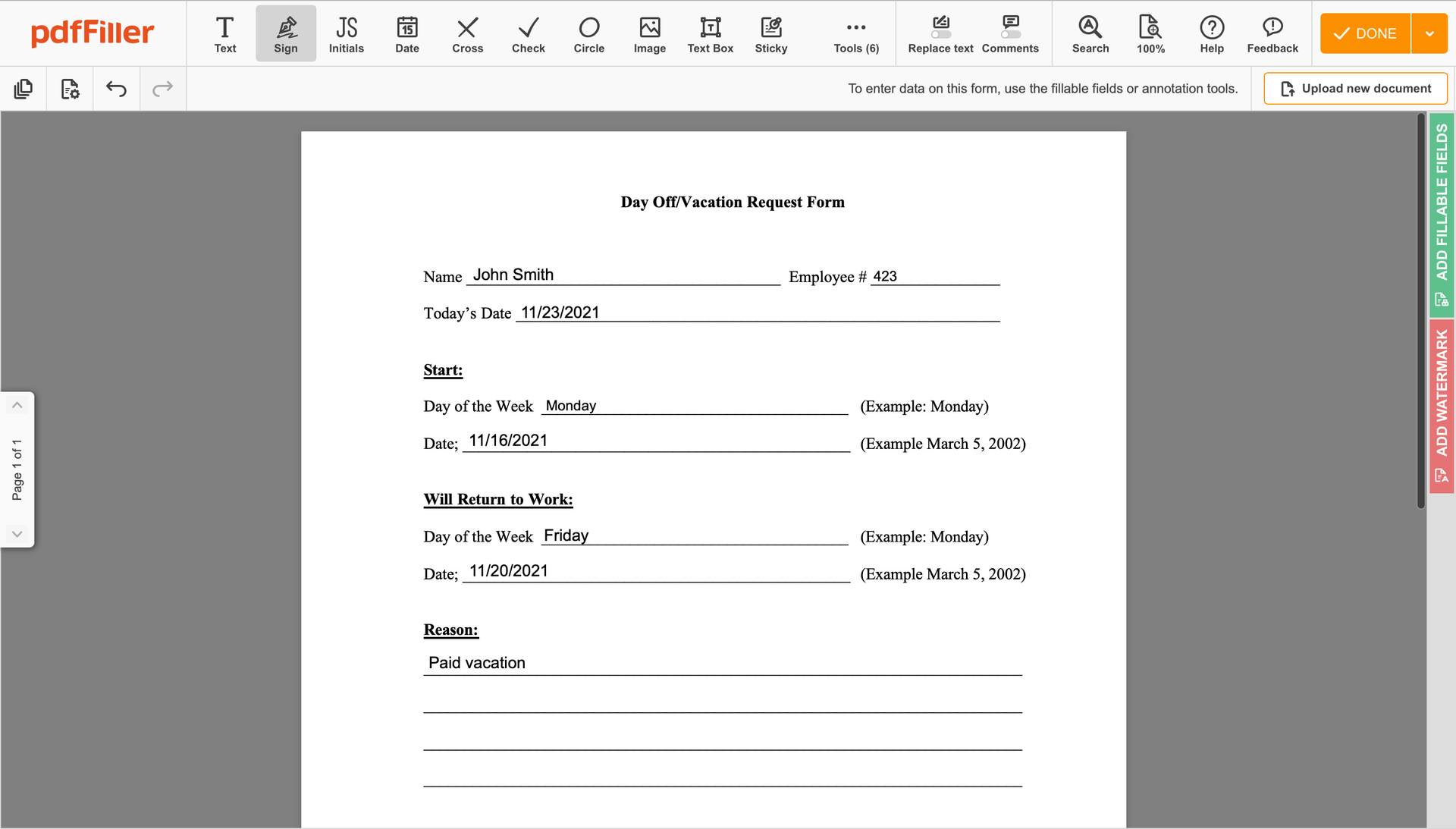
Create your electronic signature by typing, drawing, or adding your handwritten signature's image from your laptop. Then, click Save and sign.
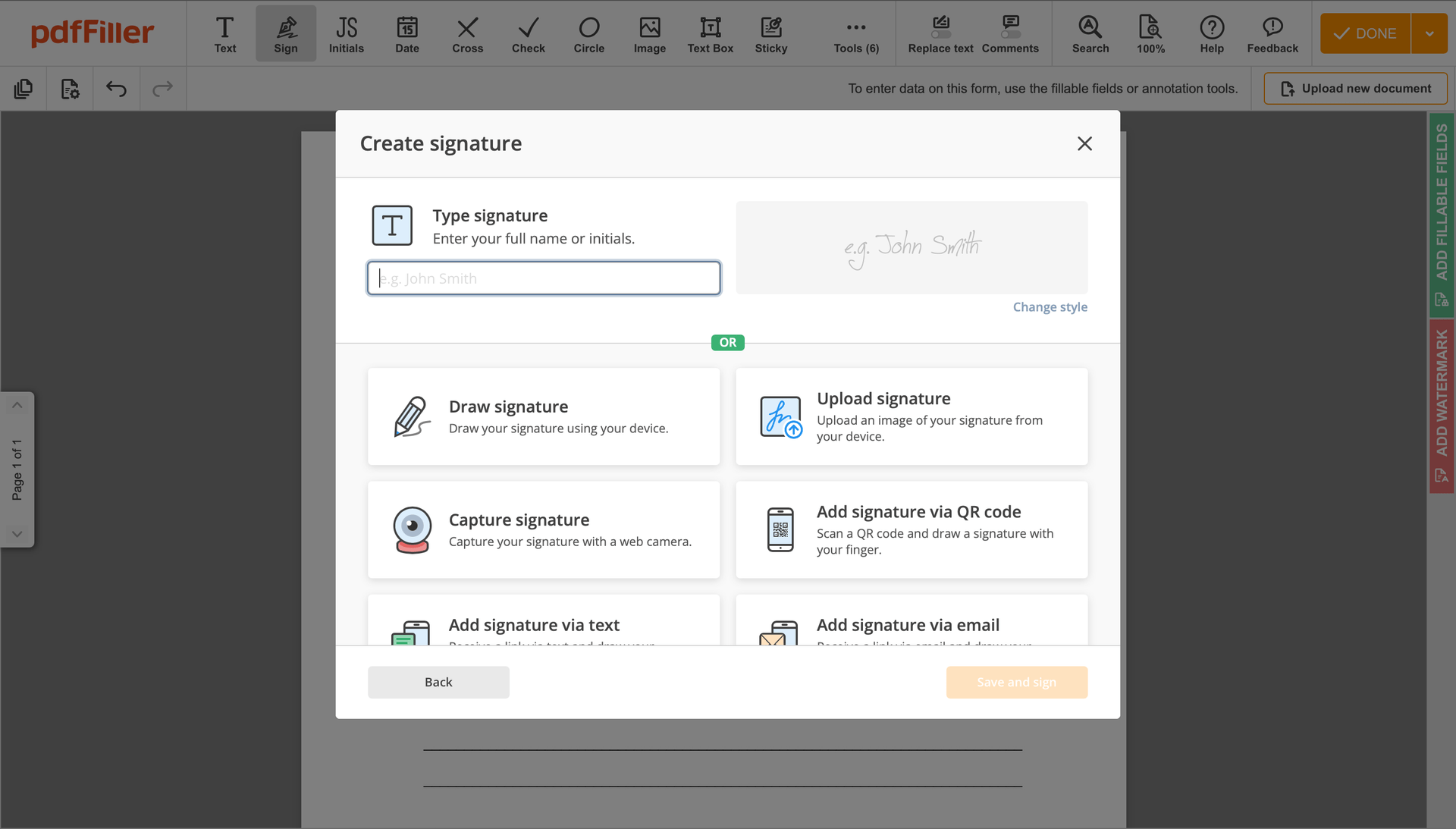
Click anywhere on a document to Redline Clinical Trial Agreement Template. You can move it around or resize it utilizing the controls in the floating panel. To use your signature, click OK.
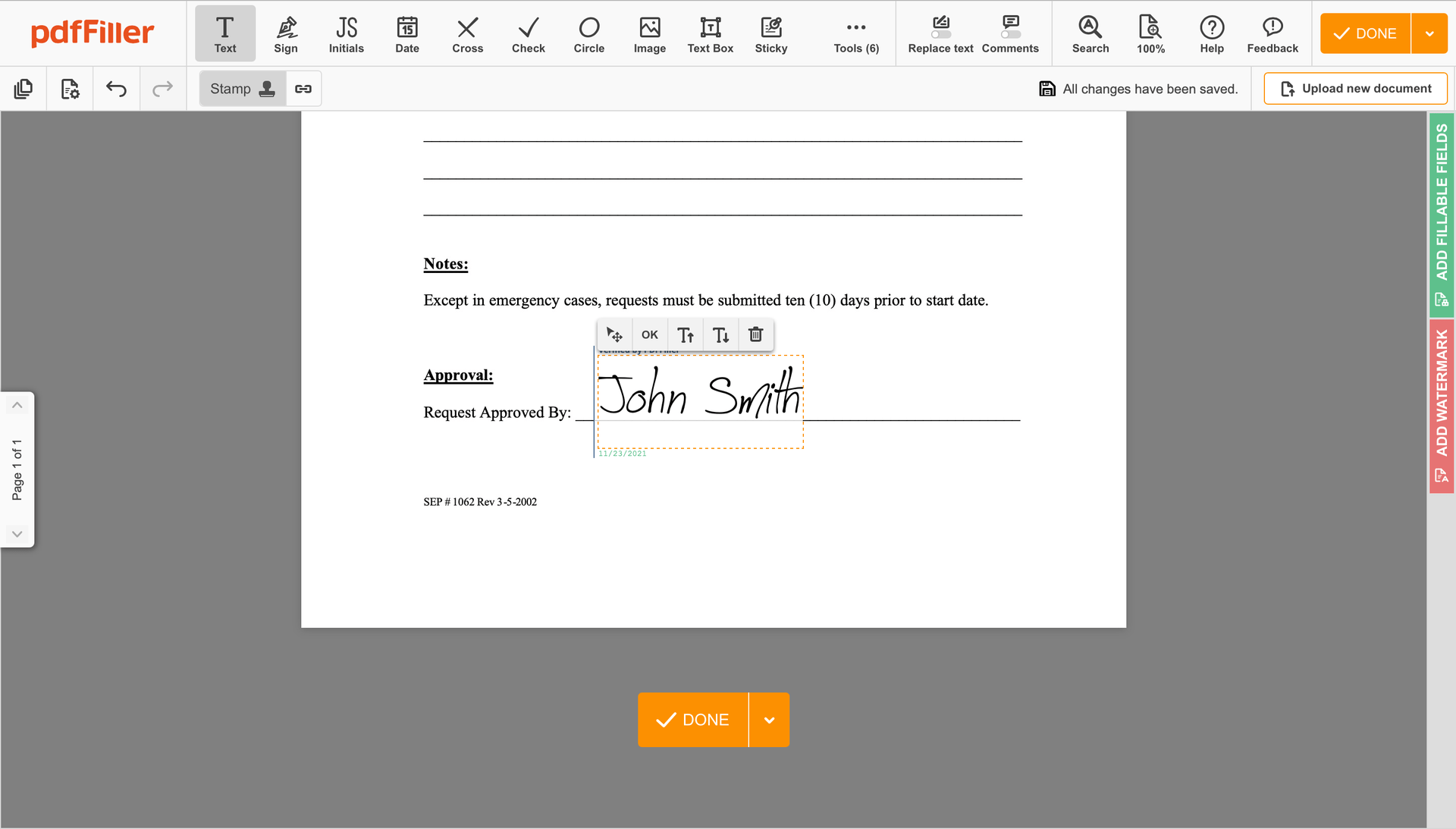
Complete the signing session by hitting DONE below your form or in the top right corner.
After that, you'll go back to the pdfFiller dashboard. From there, you can download a completed copy, print the document, or send it to other people for review or validation.
Stuck with numerous applications to manage documents? Try this all-in-one solution instead. Use our platform to make the process efficient. Create document templates completely from scratch, modify existing form sand other useful features, without leaving your browser. You can use Redline Clinical Trial Agreement Template with ease; all of our features are available instantly to all users. Have an advantage over other programs.
How to edit a PDF document using the pdfFiller editor:
How to Send a PDF for eSignature
How to Use the Redline Clinical Trial Agreement Template Feature
We understand that using the Redline Clinical Trial Agreement Template feature can be a bit overwhelming at first. But don't worry, we're here to guide you through the process step-by-step!
We hope this step-by-step guide has been helpful to you. If you have any further questions or need assistance, feel free to reach out to our support team. Happy templating!
What our customers say about pdfFiller

















